Label: ISSEEY ANTIBACTERIAL ALCOHOL WET WIPES- ethyl alcohol, benzalkonium chloride cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 80568-299-01, 80568-299-02 - Packager: ISSEEY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
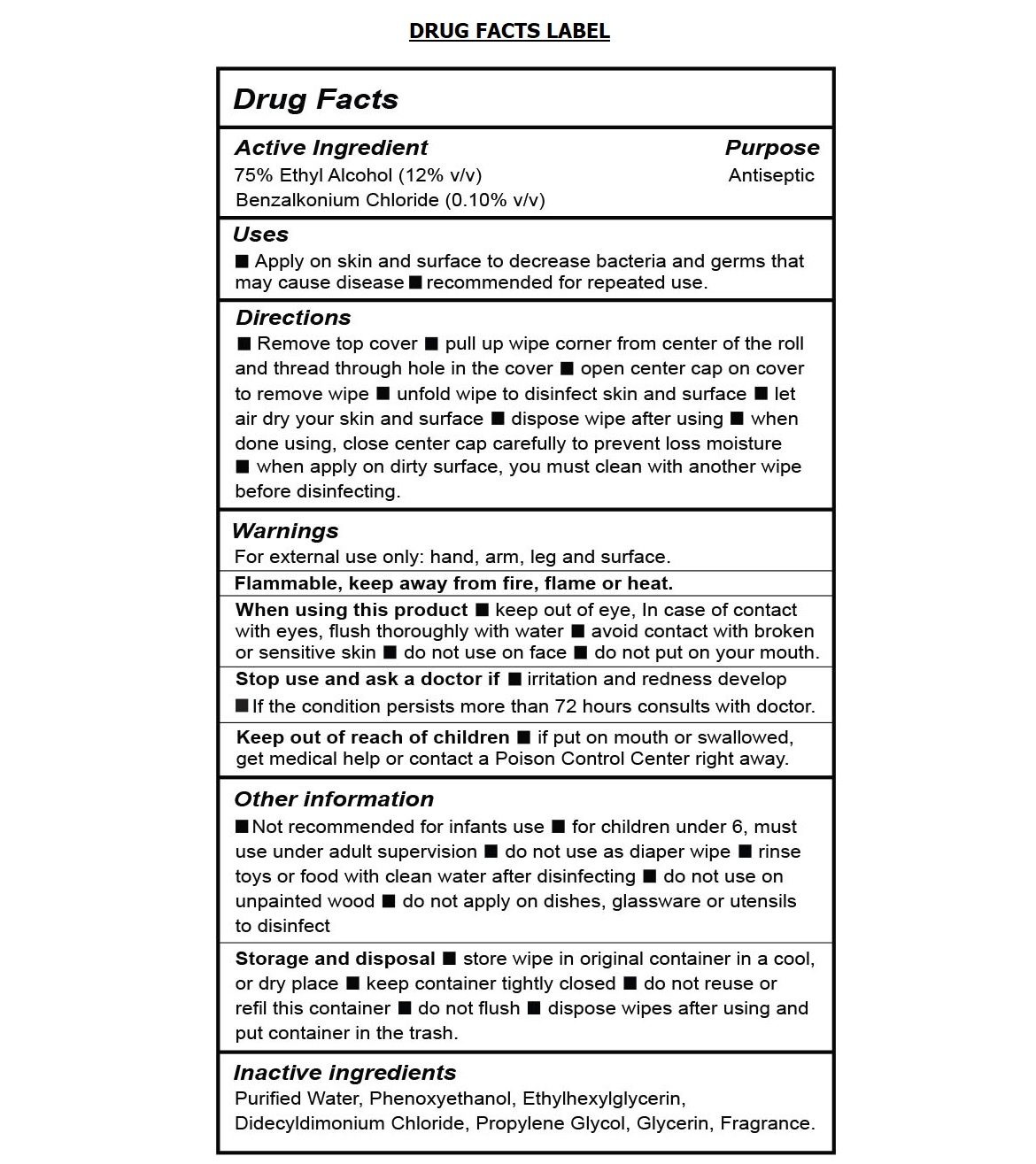
- Drug Facts
- Active Ingredient
- Purpose
- Uses
-
Directions
• Remove top cover • pull up wipe corner from center of the roll and thread through hole in the cover • open center cap on cover to remove wipe • unfold wipe to disinfect skin and surface • let air dry your skin and surface • dispose wipe after using • when done using, close center cap carefully to prevent loss moisture • when apply on dirty surface, you must clean with another wipe before disinfecting.
-
Warnings
For external use only: hand, arm, leg and surface.
Flammable, keep away from fire, flame or heat.
When using this product • keep out of eye, In case of contact with eyes, flush thoroughly with water • avoid contact with broken or sensitive skin • do not use on face • do not put on your mouth.
Stop use ask a doctor if •irritation and redness develop • If the condition persists more than 72 hours consults with doctor.
-
Other information
• Not recommended for infants use • for children under 6, must use under adult supervision • do not use as diaper wipe • rinse toys or food with clean water after disinfecting • do not use on unpainted wood • do not apply on dishes, glassware or utensils to disinfect
Storage and disposal • store wipe in original container in a cool, or dry place • keep container tightly closed • do not reuse or refill this container • do not flush • dispose wipes after using and put container in the trash.
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
KILLS 99.9% OF GERMS
CONTAINING ETHYL ALCOHOL
BLEACH FREE
Sanitize your hand and surface with wet alcohol wipes to decrease germs and bacteria. Our wipes formulated to be gentle on skin without dyes, parabens or harsh chemicals. so that it can be used safely on your hand and washable non-porous surfaces. You can clean and disinfect by wiping surface area until visible wet, allow to remain wet until air dry.
GENTLE ON HAND
BEDROOM
KITCHEN
BATHROOM
APPLIANCES
HOME OR OFFICE
MADE IN CHINA
Distributed By
ISSEEY TM
P.O. Box 2015
Alief, TX - 77411
Questions?
info.isseey@gmail.com
www.isseey.com
- Packaging
-
INGREDIENTS AND APPEARANCE
ISSEEY ANTIBACTERIAL ALCOHOL WET WIPES
ethyl alcohol, benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80568-299 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80568-299-01 100 in 1 CANISTER 09/23/2020 1 0.13 mL in 1 PACKAGE; Type 0: Not a Combination Product 2 NDC:80568-299-02 100 in 1 CANISTER 09/23/2020 2 0.13 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/23/2020 Labeler - ISSEEY (117606552)