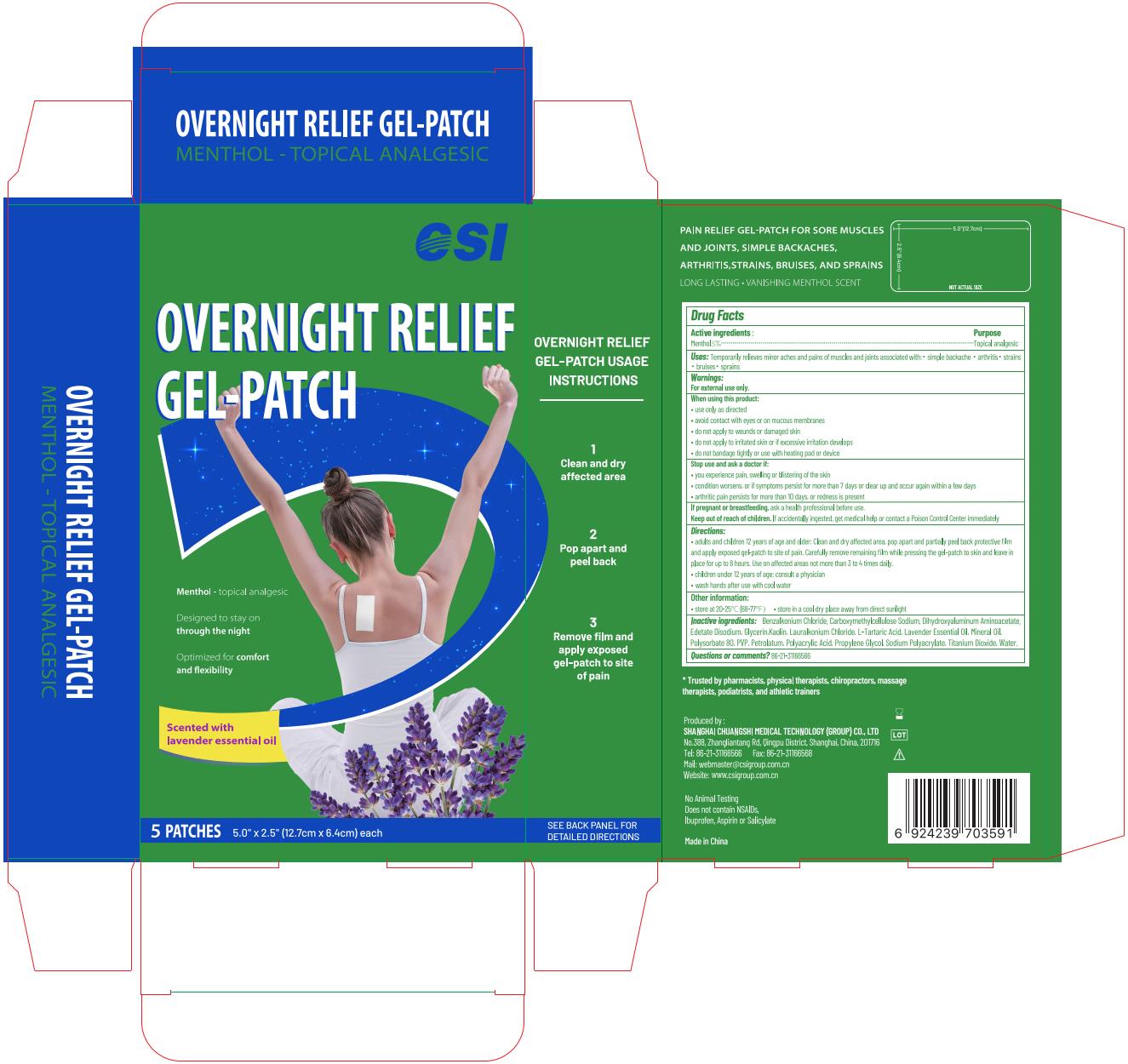
Label: OVERNIGHT RELIEF GEL-PATCH- menthol patch
- NDC Code(s): 73557-133-01, 73557-133-05
- Packager: Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this prudocts:
• use only as directed
• avoid contact with eyes or on mucous membranes
• do not apply to wounds or damaged skin
• do not apply to irritated skin or if excessive irritation develops
• do not bandage tightly or use with heating pad or deviceStop use and ask a doctor if:
• you experience pain, swelling or blistering of the skin
• condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
• arthritic pain persists for more than 10 days, or redness is presentIf pregnant or breastfeeding,ask a health professional before use.
Keep out of reach of children.If accidentally ingested, get medical help or contact a Poison Control Center immediately
-
Ask doctor
Stop use and ask a doctor if:
• you experience pain, swelling or blistering of the skin
• condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
• arthritic pain persists for more than 10 days, or redness is presentIf pregnant or breastfeeding,ask a health professional before use.
- When using
- Do not use
- Stop use
- Pregnancy or breast feeding
- Keep out of reach of children
-
Directions
• adults and children 12 years of age and older: Clean and dry affected area, pop apart and partially peel back protective film and apply exposed gel-patch to site of pain. Carefully remove remaining film while pressing the gel-patch to skin and leave in place for up to 8 hours. Use on affected areas not more than 3 to 4 times daily.
• children under 12 years of age: consult a physician
• wash hands after use with cool water - Dosage forms & strengths
-
Inactive ingredients
Water, Glycerin, Polyacrylic Acid, Propylene Glycol, Sodium Polyacrylate, Mineral Oil, Lavender Essential Oil, Polysorbate 80, PVP, Petrolatum, Dihydroxyaluminum Aminoacetate, Edetate Disodium, Kaolin, Carboxymethylcellulose Sodium, Titanium Dioxide, L-Tartaric Acid, Benzalkonium Chloride, Lauralkonium Chloride
- Questions or comments
- Other information
- Package label. Principal display panel
-
INGREDIENTS AND APPEARANCE
OVERNIGHT RELIEF GEL-PATCH
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73557-133 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.05 g in 1 g Inactive Ingredients Ingredient Name Strength KAOLIN (UNII: 24H4NWX5CO) 0.001 g in 1 g TARTARIC ACID (UNII: W4888I119H) 0.001 g in 1 g WATER (UNII: 059QF0KO0R) 0.3095 g in 1 g DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) 0.0015 g in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.06 g in 1 g POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) 0.2 g in 1 g CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 0.001 g in 1 g SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) 0.05 g in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.001 g in 1 g MINERAL OIL (UNII: T5L8T28FGP) 0.03 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 0.002 g in 1 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.001 g in 1 g LAURALKONIUM CHLORIDE (UNII: 07HUP5A29X) 0.001 g in 1 g POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.018 g in 1 g GLYCERIN (UNII: PDC6A3C0OX) 0.24 g in 1 g POVIDONE K90 (UNII: RDH86HJV5Z) 0.012 g in 1 g LAVENDER OIL (UNII: ZBP1YXW0H8) 0.02 g in 1 g EDETATE DISODIUM (UNII: 7FLD91C86K) 0.001 g in 1 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73557-133-05 5 in 1 BOX 12/30/2022 1 NDC:73557-133-01 8 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/30/2022 Labeler - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) Registrant - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) Establishment Name Address ID/FEI Business Operations Shanghai Chuangshi Medical Technology (Group) Co., Ltd. 546872672 manufacture(73557-133) , label(73557-133)