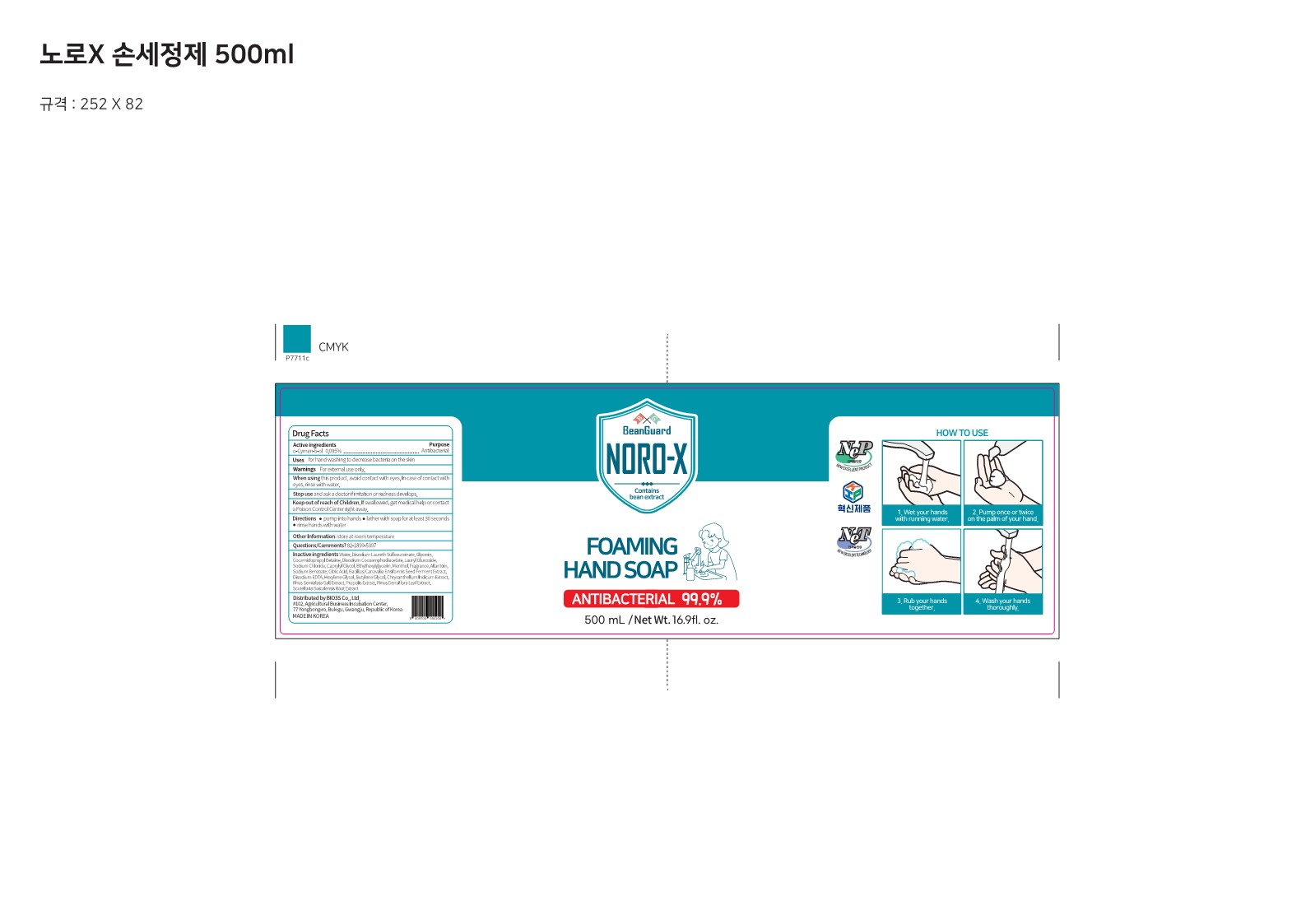
Label: NORO-X FOAMING HANDSOAP- o-cymen-5-ol soap
- NDC Code(s): 77935-507-01
- Packager: BIO3S Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purposes
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
-
Inactive ingredients
Water, Disodium Laureth Sulfosuccinate, Glycerin, Cocamidopropyl Betaine, Disodium Cocoamphodiacetate, Lauryl Glucoside,
Sodium Chloride, Capiylyi Glycol, Ethyihexylglycerin, Menthol, Fragrance, Allantoin, Sodium Benzoate, Citric Acid, Bacillus/Canavalia Ensiformis Seed Ferment Extract, Disodium EDTA, Hexylene Glycol, Butylene Glycol, Chrysanthellum Indicum Extract,
RhusSemialata Gall Extract, Propolis Extract, Pinus Densiflora Leaf Extract, Scutellaria Baicalensis Root Extract - Display Panel
-
INGREDIENTS AND APPEARANCE
NORO-X FOAMING HANDSOAP
o-cymen-5-ol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77935-507 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength O-CYMEN-5-OL (UNII: H41B6Q1I9L) (O-CYMEN-5-OL - UNII:H41B6Q1I9L) O-CYMEN-5-OL 0.095 g in 100 mL Inactive Ingredients Ingredient Name Strength DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM BENZOATE (UNII: OJ245FE5EU) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CHRYSANTHELLUM INDICUM TOP (UNII: STJ856D1Z0) MENTHOL (UNII: L7T10EIP3A) ALLANTOIN (UNII: 344S277G0Z) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PINUS DENSIFLORA LEAF (UNII: Q1Q9P50WIY) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) PROPOLIS WAX (UNII: 6Y8XYV2NOF) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) RHUS CHINENSIS GALL (UNII: 4W3Y2V7J3R) CANAVALIA ENSIFORMIS WHOLE (UNII: U485ST9OUN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77935-507-01 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/27/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/27/2022 Labeler - BIO3S Co.,Ltd. (694813103) Registrant - BIO3S Co.,Ltd. (694813103) Establishment Name Address ID/FEI Business Operations BIO3S Co.,Ltd. 694813103 manufacture(77935-507)