Label: NATURAL WHITE RADIANT WHITE- fluoride paste, dentifrice
- NDC Code(s): 62721-0473-9
- Packager: Lornamead
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Adults and children 2 years of age and older: brush teeth thorughly preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to avoid swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 12 years of age: consult a dentist or a doctor.
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
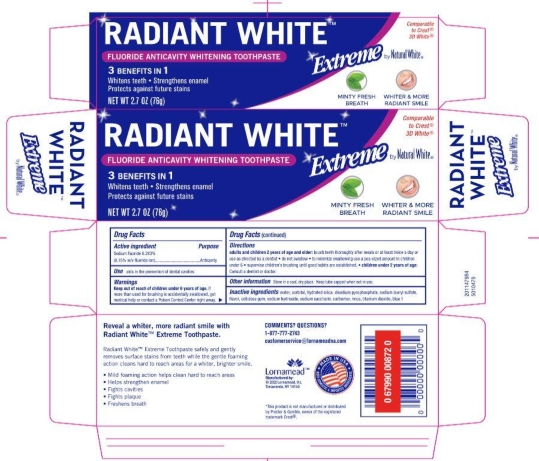
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATURAL WHITE RADIANT WHITE
fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62721-0473 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.15 g in 100 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM ACID PYROPHOSPHATE (UNII: H5WVD9LZUD) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MICA (UNII: V8A1AW0880) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color blue (light blue with sparkles) Score Shape Size Flavor MINT (mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62721-0473-9 1 in 1 CARTON 01/01/2013 1 76 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/01/2013 Labeler - Lornamead (126440440) Registrant - Lornamead (080046418) Establishment Name Address ID/FEI Business Operations Lornamead Inc. 080046418 manufacture(62721-0473) , pack(62721-0473)