Label: MIGRAINE BLOCKER (chamomilla, bryonia (alba), iris versicolor, juglans cinerea, pulsatilla- pratensis, sanguinaria canadensis, magnesia muriatica, natrum muriaticum, phosphorus, silicea, zincum metallicum, belladonna, gelsemium sempervirens, ignatia amara, scutellaria lateriflora tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 64471-807-48 - Packager: Source Naturals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 18, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
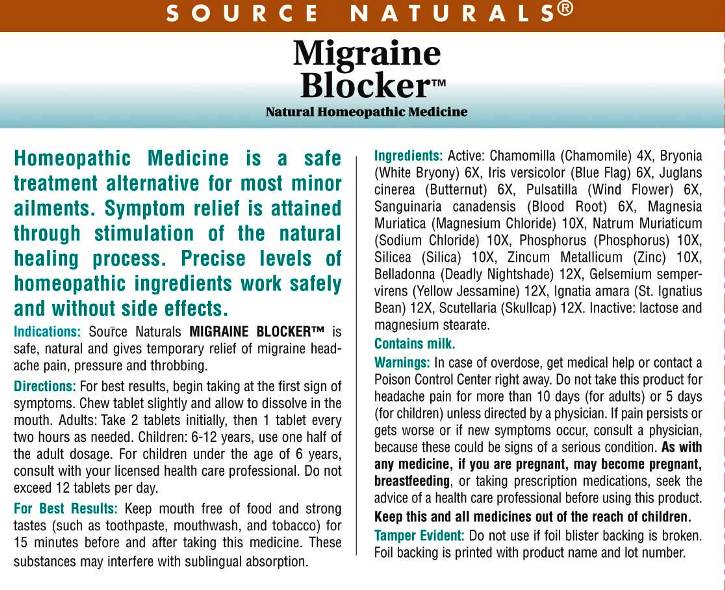
ACTIVE INGREDIENTS:
Chamomilla 4X, Bryonia (Alba) 6X, Iris Versicolor 6X, Juglans Cinerea 6X, Pulsatilla (Pratensis) 6X, Sanguinaria Canadensis 6X, Magnesia Muriatica 10X, Natrum Muriaticum 10X, Phosphorus 10X, Silicea 10X, Zincum Metallicum 10X, Belladonna 12X, Gelsemium Sempervirens 12X, Ignatia Amara 12X, Scutellaria Lateriflora 12X.
- INDICATIONS:
-
WARNINGS:
In case of overdose, get medical help or contact a Poison Control Center right away.
Do not take this product for headache pain for more than 10 days (for adults) or 5 days (for children) unless directed by a physician.
If pain persists or gets worse or if new symptoms occur, consult a physician, because these could be signs of a serious condition.
As with any medicine, if you are pregnant, may become pregnant, or breastfeeding, or taking prescription medications, seek the advice of a health care professional before using this product.
Keep this and all medicines out of the reach of children.
Tamper Evident: Do not use if foil blister backing is broken. Foil backing is printed with product name and lot number.
- KEEP OUT OF REACH OF CHILDREN:
-
DIRECTIONS:
For best results, begin taking at the first sign of symptoms. Chew tablet slightly and allow to dissolve in the mouth.
Adults: Take 2 tablets, then 1 tablet every two hours as needed. Children: 6-12 years, use one half of the adult dosage.
For children under the age of 6 years, consult with your licensed health care professional.
Do not exceed 12 tablets per day.
For Best Results: Keep mouth free of food and strong tastes (such as toothpaste, mouthwash and tobacco) for 15 minutes before and after taking this medicine. These substances may interfere with sublingual absorption.
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
MIGRAINE BLOCKER
chamomilla, bryonia (alba), iris versicolor, juglans cinerea, pulsatilla (pratensis), sanguinaria canadensis, magnesia muriatica, natrum muriaticum, phosphorus, silicea, zincum metallicum, belladonna, gelsemium sempervirens, ignatia amara, scutellaria lateriflora tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64471-807 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 4 [hp_X] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] IRIS VERSICOLOR ROOT (UNII: X43D4L3DQC) (IRIS VERSICOLOR ROOT - UNII:X43D4L3DQC) IRIS VERSICOLOR ROOT 6 [hp_X] JUGLANS CINEREA BRANCH BARK/ROOT BARK (UNII: 48FZ1BHO18) (JUGLANS CINEREA BRANCH BARK/ROOT BARK - UNII:48FZ1BHO18) JUGLANS CINEREA BRANCH BARK/ROOT BARK 6 [hp_X] PULSATILLA PRATENSIS (UNII: 8E272251DI) (PULSATILLA PRATENSIS - UNII:8E272251DI) PULSATILLA PRATENSIS 6 [hp_X] SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 6 [hp_X] MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 10 [hp_X] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 10 [hp_X] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 10 [hp_X] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 10 [hp_X] ZINC (UNII: J41CSQ7QDS) (ZINC - UNII:J41CSQ7QDS) ZINC 10 [hp_X] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 12 [hp_X] SCUTELLARIA LATERIFLORA (UNII: 7BP4DH5PDC) (SCUTELLARIA LATERIFLORA - UNII:7BP4DH5PDC) SCUTELLARIA LATERIFLORA 12 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND (TABLET) Size 10mm Flavor Imprint Code H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64471-807-48 2 in 1 CARTON 11/18/2016 1 24 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/18/2016 Labeler - Source Naturals, Inc. (969024228) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(64471-807) , api manufacture(64471-807) , label(64471-807) , pack(64471-807)