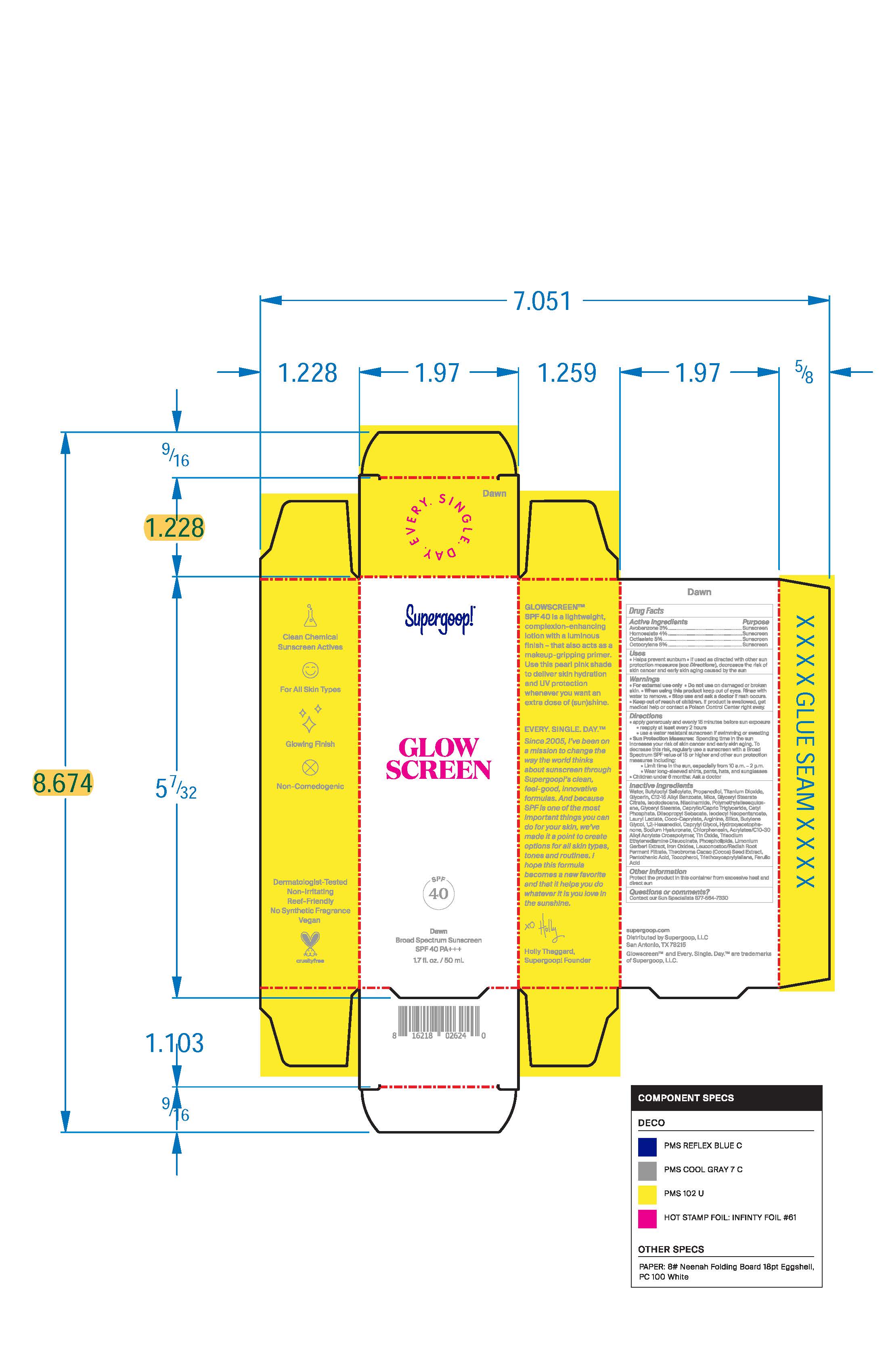
Label: GLOWSCREEN SPF 40 - DAWN- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 75936-606-01, 75936-606-02, 75936-606-03
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Directions
Apply generously and evenly 15 minutes before sun exposure
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m.- 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor
-
INACTIVE INGREDIENT
Water, Butyloctyl Salicylate, Propanediol, Titanium Dioxide, Glycerin, C12-15 Alkyl Benzoate, Mica, Glyceryl Stearate Citrate, Isododecane, Niacinamide, Polymethylsilsesquioxane, Glyceryl Stearate, Caprylic/Capric Triglyceride, Cetyl Phosphate, Diisopropyl Sebacate, Isodecyl Neopentanoate, Lauryl Lactate, Coco-Caprylate, Arginine, Silica, Butylene Glycol, 1,2-Hexanediol, Caprylyl Glycol, Hydroxyacetophenone, Sodium Hyaluronate, Chlorphenesin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tin Oxide, Trisodium Ethylenediamine Disuccinate, Phospholipids, Limonium Gerberi Extract, Iron Oxides, Leuconostoc/Radish Root Ferment Filtrate, Theobroma Cacao (Cocoa) Seed Extract, Pantothenic Acid, Tocopherol, Triethoxycaprylylsilane, Ferulic Acid
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GLOWSCREEN SPF 40 - DAWN
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-606 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8 g in 100 mL Inactive Ingredients Ingredient Name Strength BROWN IRON OXIDE (UNII: 1N032N7MFO) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) LIMONIUM GERBERI FLOWERING TOP (UNII: V4O4C05BJ2) WATER O-18 (UNII: 7QV8F8BYNJ) CAPRYLIC/CAPRIC/PALMITIC/STEARIC TRIGLYCERIDE (UNII: ZF29F7IK5I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) ISODODECANE (UNII: A8289P68Y2) GLYCERIN (UNII: PDC6A3C0OX) COCO-CAPRYLATE (UNII: 4828G836N6) PANTOTHENIC ACID (UNII: 19F5HK2737) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERULIC ACID (UNII: AVM951ZWST) NIACINAMIDE (UNII: 25X51I8RD4) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) STANNIC OXIDE (UNII: KM7N50LOS6) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) PEG-120 GLYCERYL STEARATE (UNII: 6941286E4I) LAURYL LACTATE (UNII: G5SU0BFK7O) PROPANEDIOL (UNII: 5965N8W85T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) SILICA, TRIMETHYLSILYL CAPPED (UNII: VU10KU4B9S) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GR-270773 PHOSPHOLIPID EMULSION (UNII: D4B2F53PBH) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) CHLORPHENESIN (UNII: I670DAL4SZ) CETYL PHOSPHATE (UNII: VT07D6X67O) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MICA (UNII: V8A1AW0880) ARGININE (UNII: 94ZLA3W45F) TOCOPHEROL (UNII: R0ZB2556P8) ACRYLATES CROSSPOLYMER-6 (UNII: 4GXD0Q3OS3) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-606-02 1 in 1 CARTON 03/17/2023 1 NDC:75936-606-01 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:75936-606-03 10 mL in 1 TUBE; Type 0: Not a Combination Product 03/17/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 03/15/2023 Labeler - Supergoop, LLC (117061743)