Label: TOTY ILUMINA CC CREAMY COMPACT REFILL 4C- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 3C- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 3W- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 4N- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 1C- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 2W- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 5W1- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 5W2- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 3N- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 1N- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 1W- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 2N- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 2C- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 4W- titanium dioxide cream
TOTY ILUMINA CC CREAMY COMPACT REFILL 5W- titanium dioxide cream
-
NDC Code(s):
82141-2357-1,
82141-2358-1,
82141-2359-1,
82141-2360-1, view more82141-2361-1, 82141-2362-1, 82141-2363-1, 82141-2364-1, 82141-2365-1, 82141-2366-1, 82141-2367-1, 82141-2368-1, 82141-2369-1, 82141-2370-1, 82141-2371-1
- Packager: Chipican LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Apply liberally 15 minutes behore sun exposure
- Reapply:
After 80 minutes of swimming or sweating
immediately after towel drying
Atleat every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses
Children under 6 months of age: Ask a doctor. - Other Information
-
Inactive Ingredients
NDCs 82141-2357-1, 82141-2358-1, 82141-2360-1, 82141-2363-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax;
Polymethyl Methacrylate; Mica; Butylene Glycol Cocoate; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDCs 82141-2359-1, 82141-2362-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Mica; Hydrogenated Microcrystalline
Wax; Polymethyl Methacrylate; Butylene Glycol Cocoate; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDCs 82141-2361-1, 82141-2365-1, 82141-2368-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax;
Polymethyl Methacrylate; Butylene Glycol Cocoate; Mica; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDC 82141-2364-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax; Mica;
Polymethyl Methacrylate; Butylene Glycol Cocoate; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica;
Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Triethoxycaprylylsilane; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDC 82141-2366-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Mica; Polymethyl Methacrylate;
Hydrogenated Microcrystalline Wax; Butylene Glycol Cocoate; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDC 82141-2367-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax;
Polymethyl Methacrylate; Mica; Butylene Glycol Cocoate; Methyl Methacrylate Crosspolymer; Carnauba (Copernica cerifera) Wax; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Triethoxycaprylylsilane; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDCs 82141-2369-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax;
Butylene Glycol Cocoate; Polymethyl Methacrylate; Mica; Carnauba (Copernica cerifera) Wax; Methyl Methacrylate Crosspolymer; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Triethoxycaprylylsilane; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
NDCs 82141-2370-1, 82141-2371-1
Hydrogenated Polyisobutene; Ethylhexyl Palmitate; Nylon-12; Paraffin; Hydrogenated Microcrystalline Wax;
Butylene Glycol Cocoate; Mica; Polymethyl Methacrylate; Carnauba (Copernica cerifera) Wax; Methyl Methacrylate Crosspolymer; Silica; Hydrogenated Castor Oil; Alumina, Squalene, Calaguala (Polypodium leucotomos) Leaf Extract; Propylene Glycol; Glyceryl Caprylate; Tocopheryl Acetate; Castor (Ricinus communis) Seed Oil; Water; Simethicone; Tocopherol; Beta-Sitosterol; Soybean (Glycine soja) Oil; Triethoxycaprylylsilane; Paraffinum Liquidum; Fragrance, Linalool, Iron Oxides* (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77492), Ferrosoferric Oxide (CI 77499)); Titanium Dioxide* (CI 77891), Bismuth Oxychloride* (CI 77163). * Contains one or more of these ingredients.
-
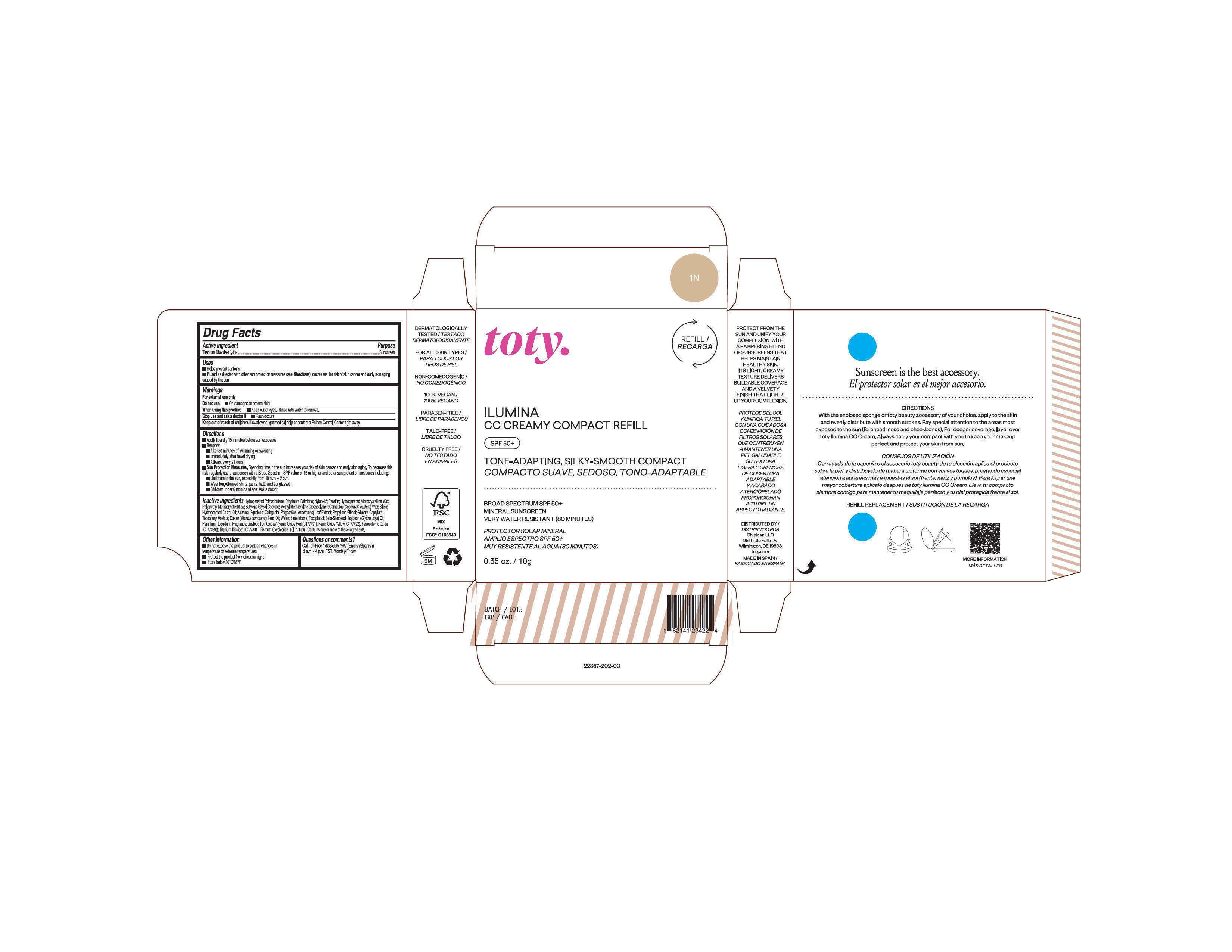
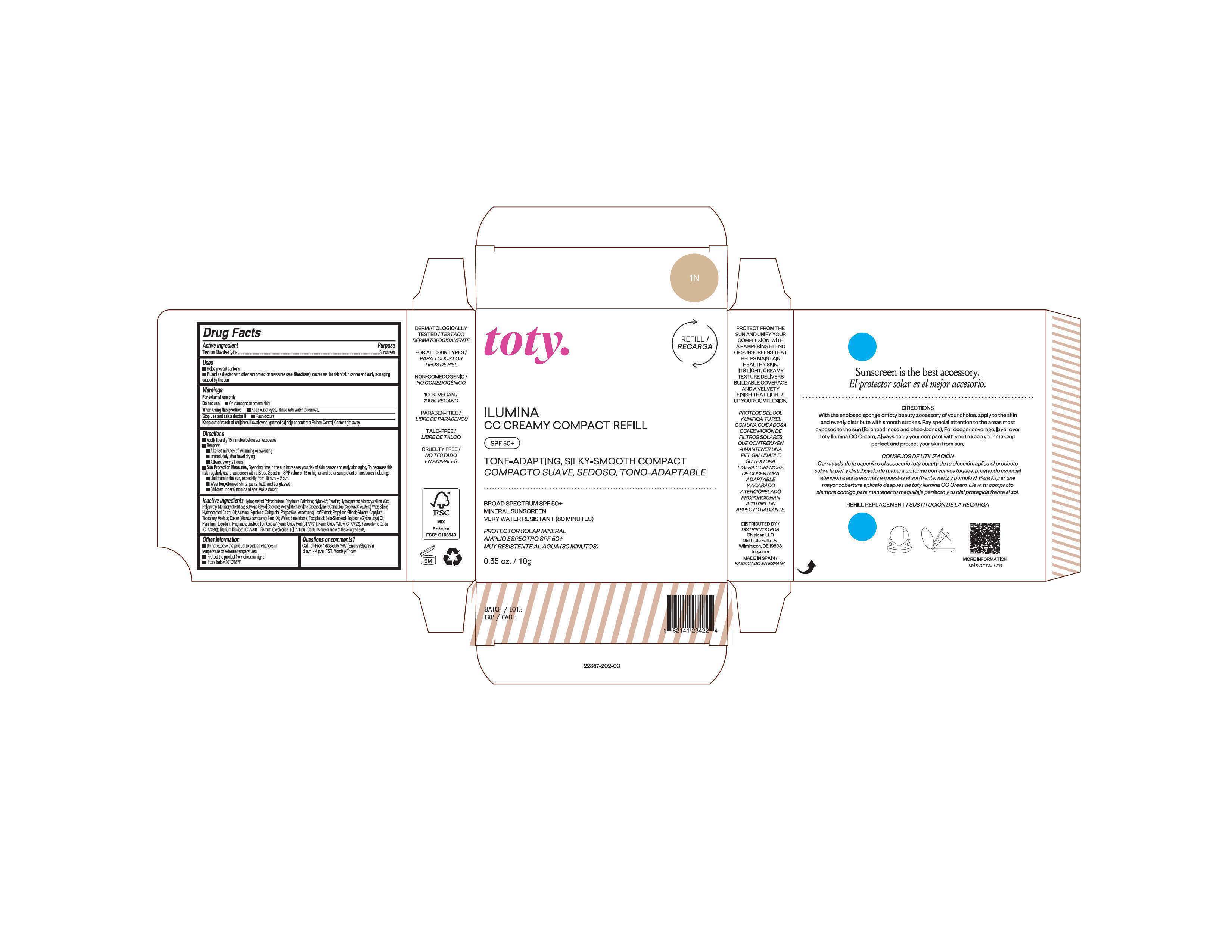
Package Label.Principal Display Panel-
1N
NDC 82141-2357-1 (toty Ilumina CC Creamy Compact Refill 1N)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
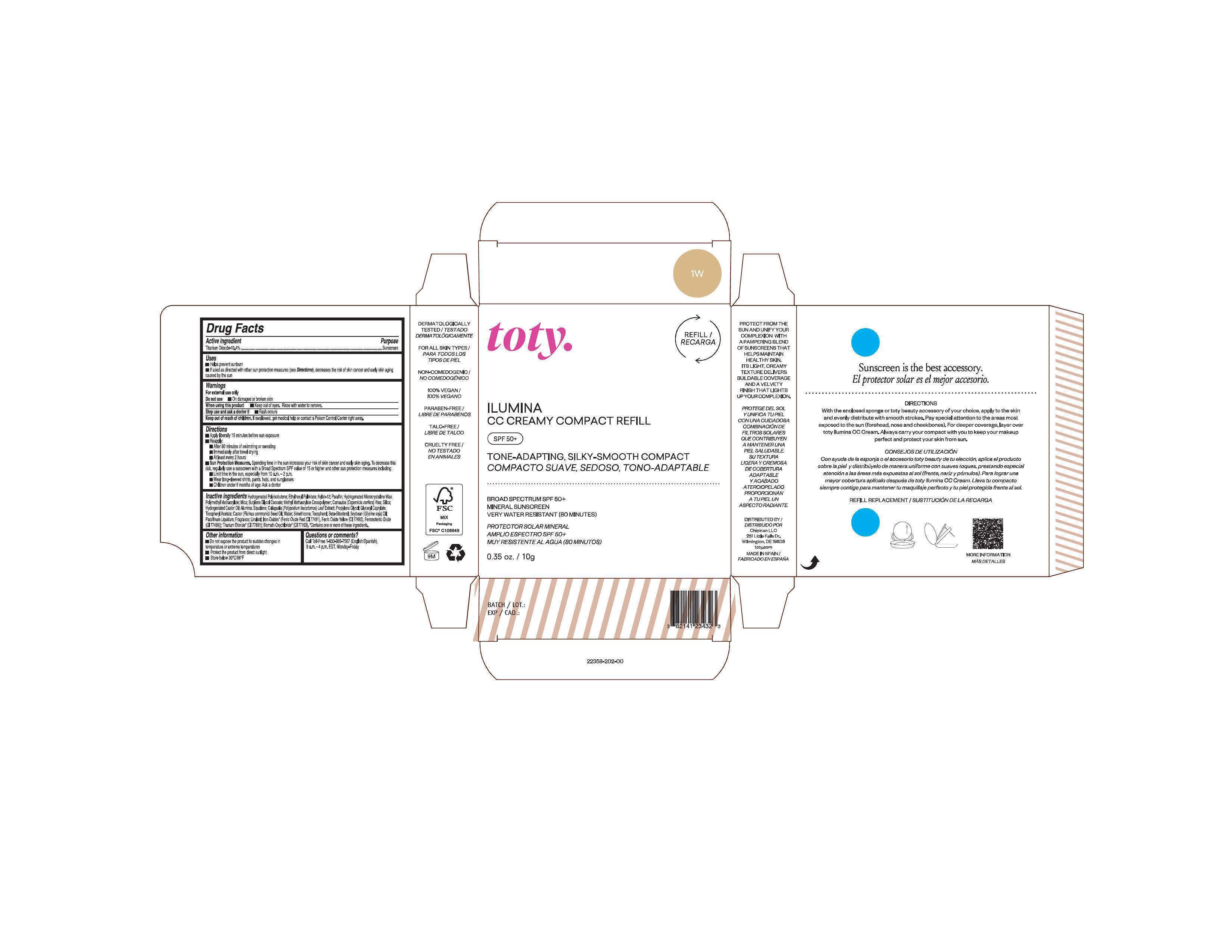
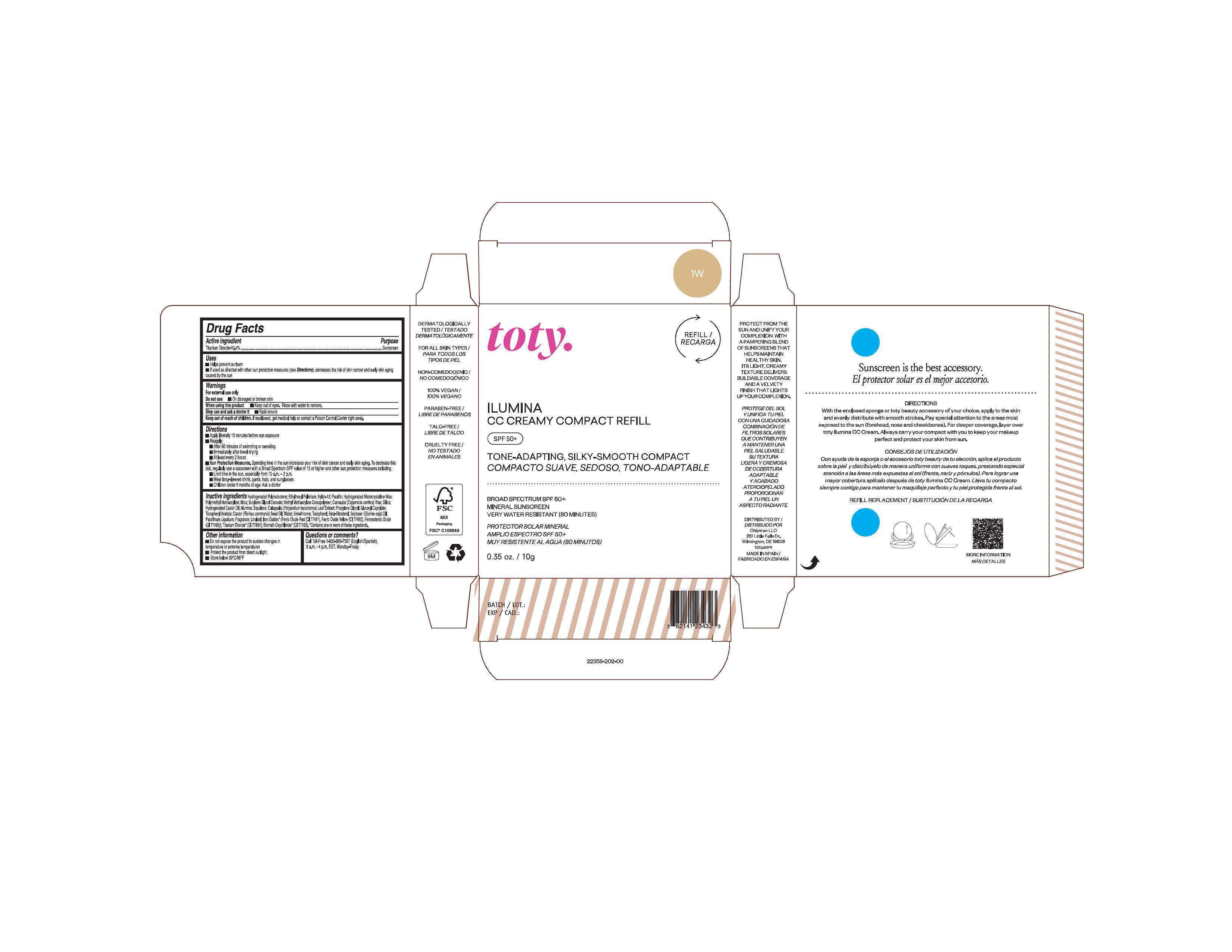
Package Label.Principal Display Panel-
1W
NDC 82141-2358-1 (toty Ilumina CC Creamy Compact Refill 1W)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
Package Label.Principal Display Panel-
1C
NDC 82141-2359-1 (toty Ilumina CC Creamy Compact Refill 1C)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
Package Label.Principal Display Panel-
2N
NDC 82141-2360-1 (toty Ilumina CC Creamy Compact Refill 2N)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
Package Label.Principal Display Panel-
2W
NDC 82141-2361-1 (toty Ilumina CC Creamy Compact Refill 2W)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
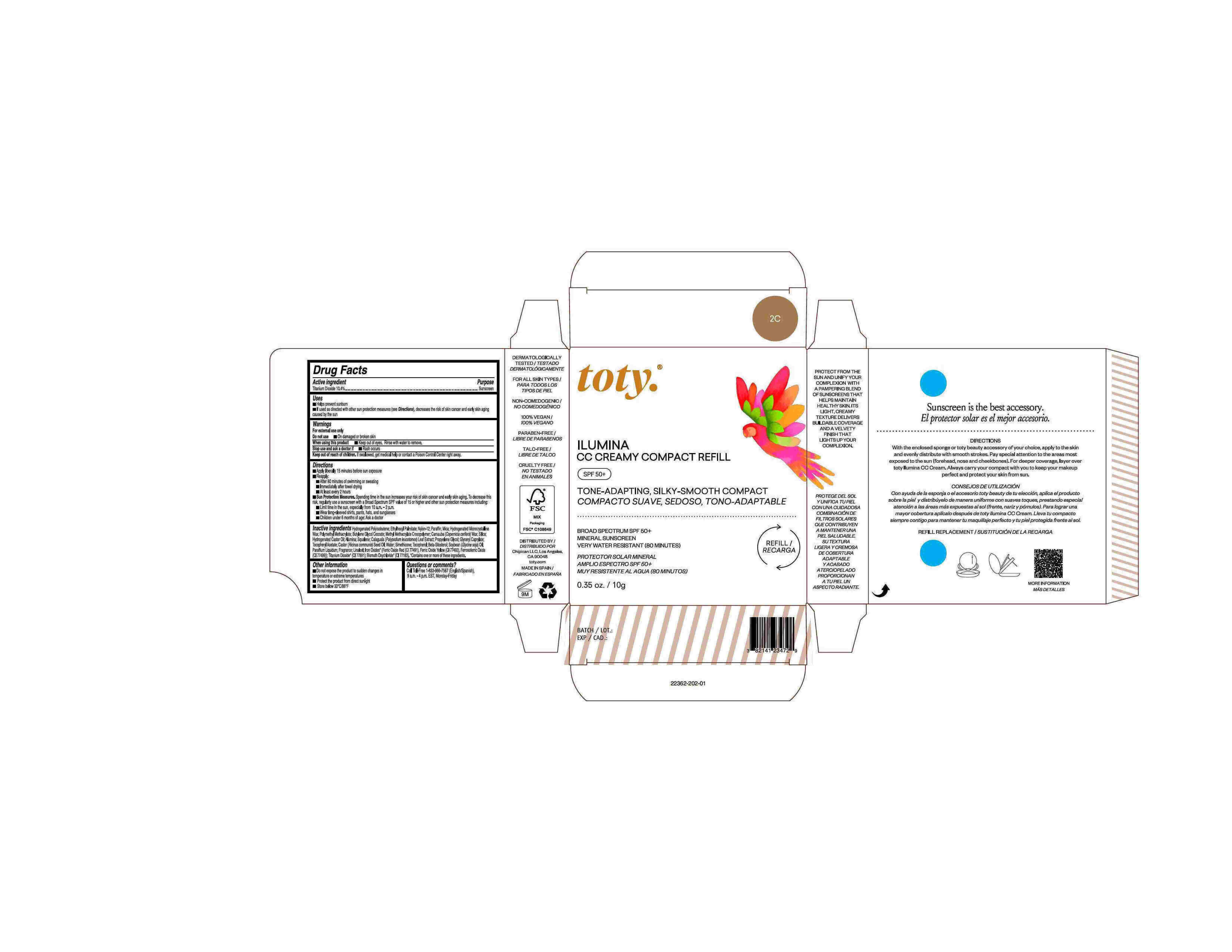
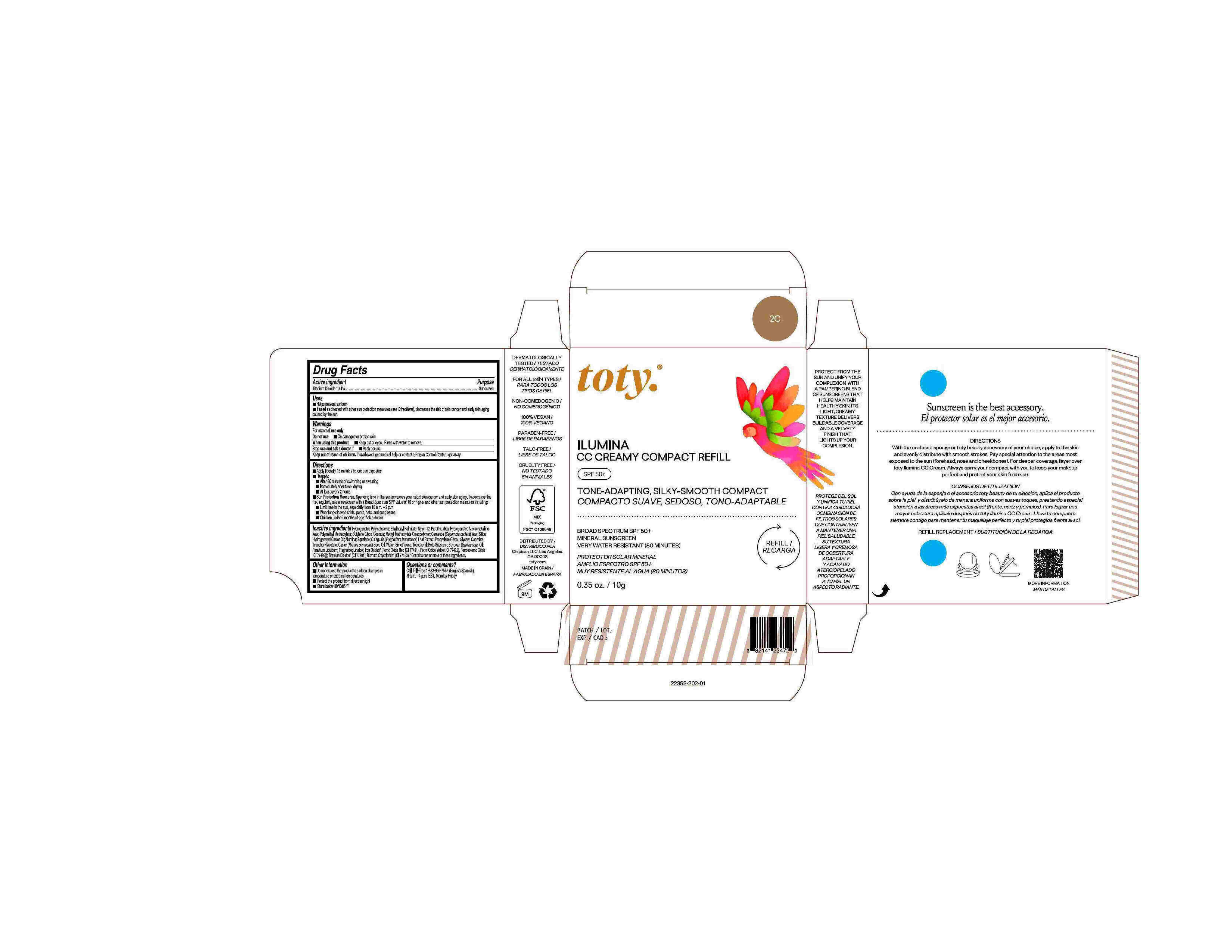
Package Label.Principal Display Panel-
2C
NDC 82141-2362-1 (toty Ilumina CC Creamy Compact Refill 2C)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
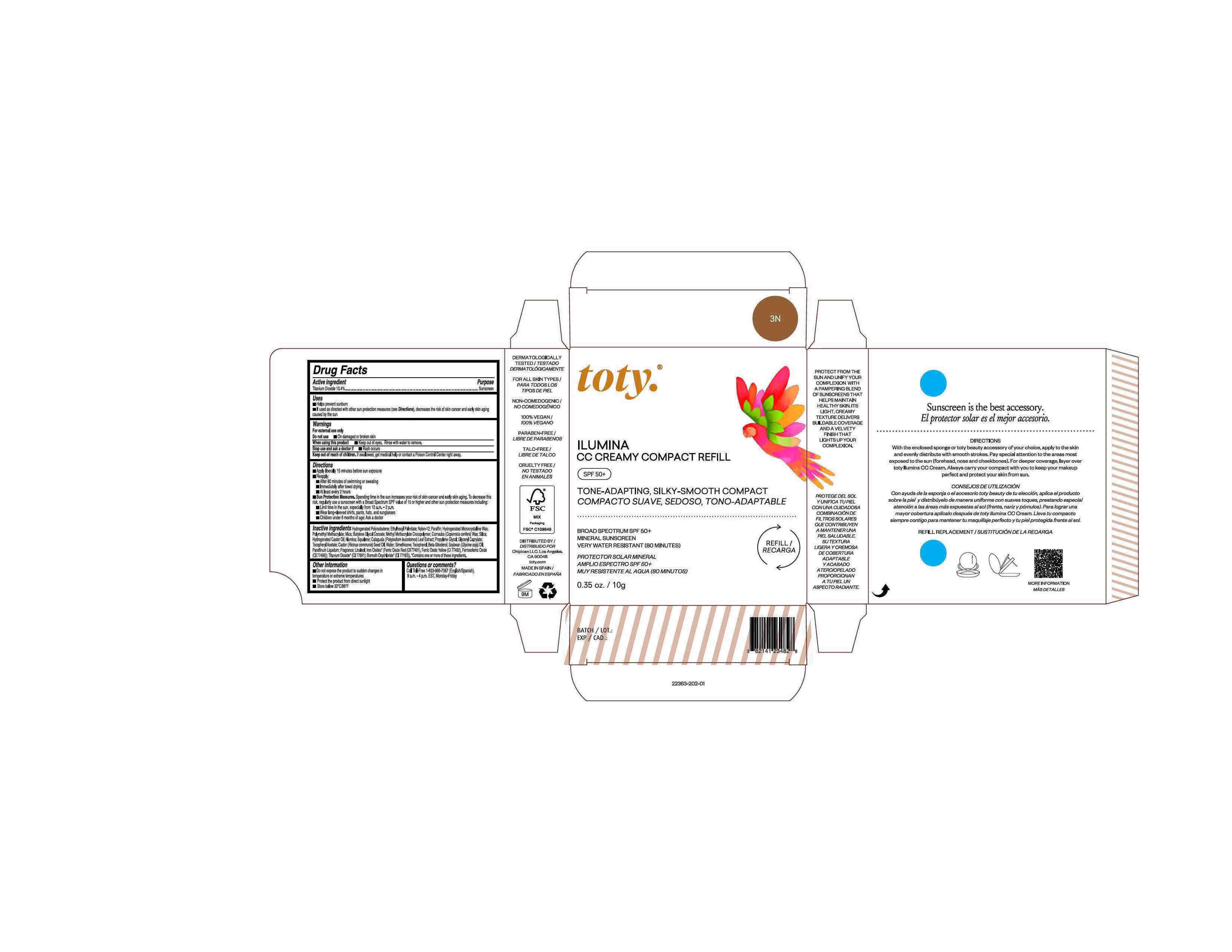
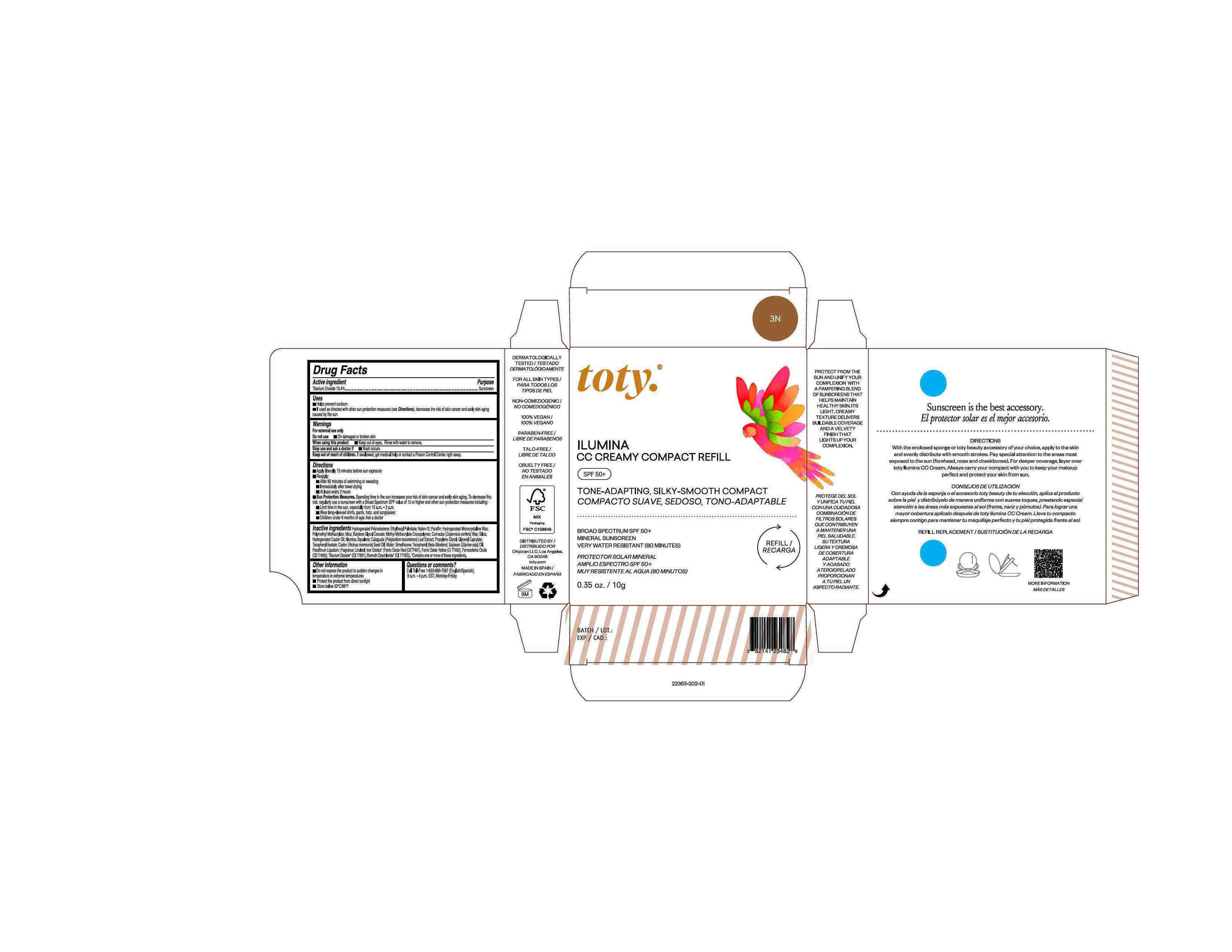
Package Label.Principal Display Panel-
3N
NDC 82141-2363-1 (toty Ilumina CC Creamy Compact Refill 3N)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
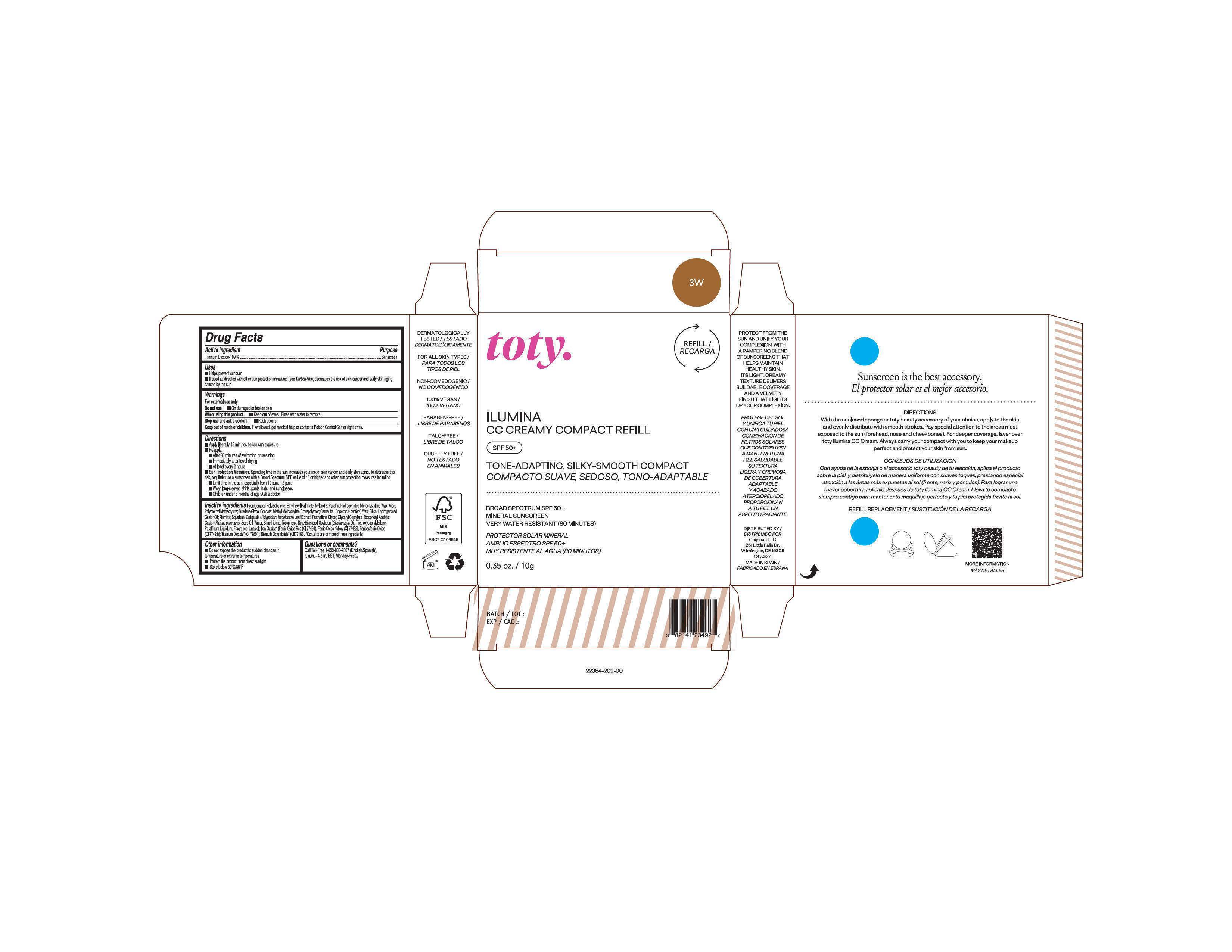
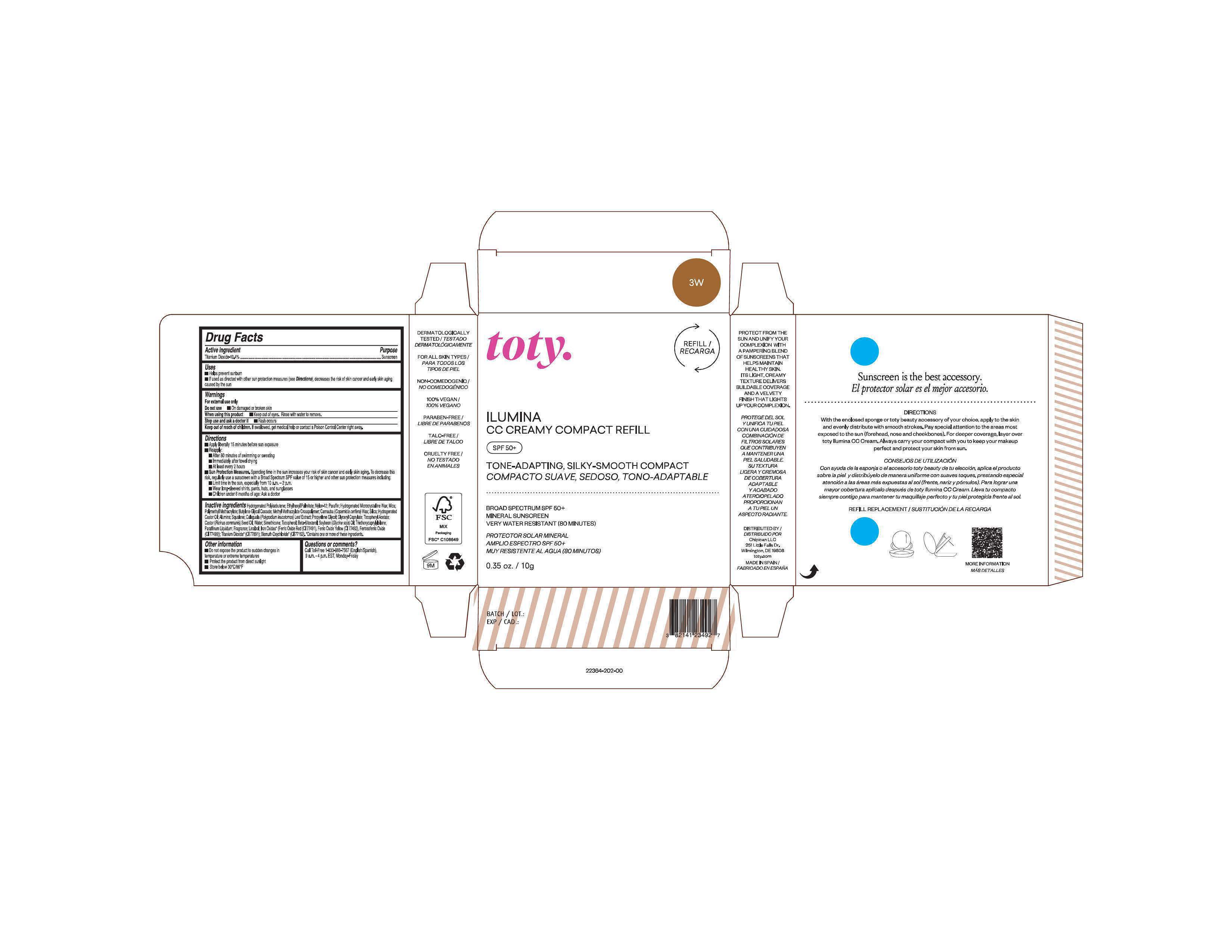
Package Label.Principal Display Panel-
3W
NDC 82141-2364-1 (toty Ilumina CC Creamy Compact Refill 3W)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
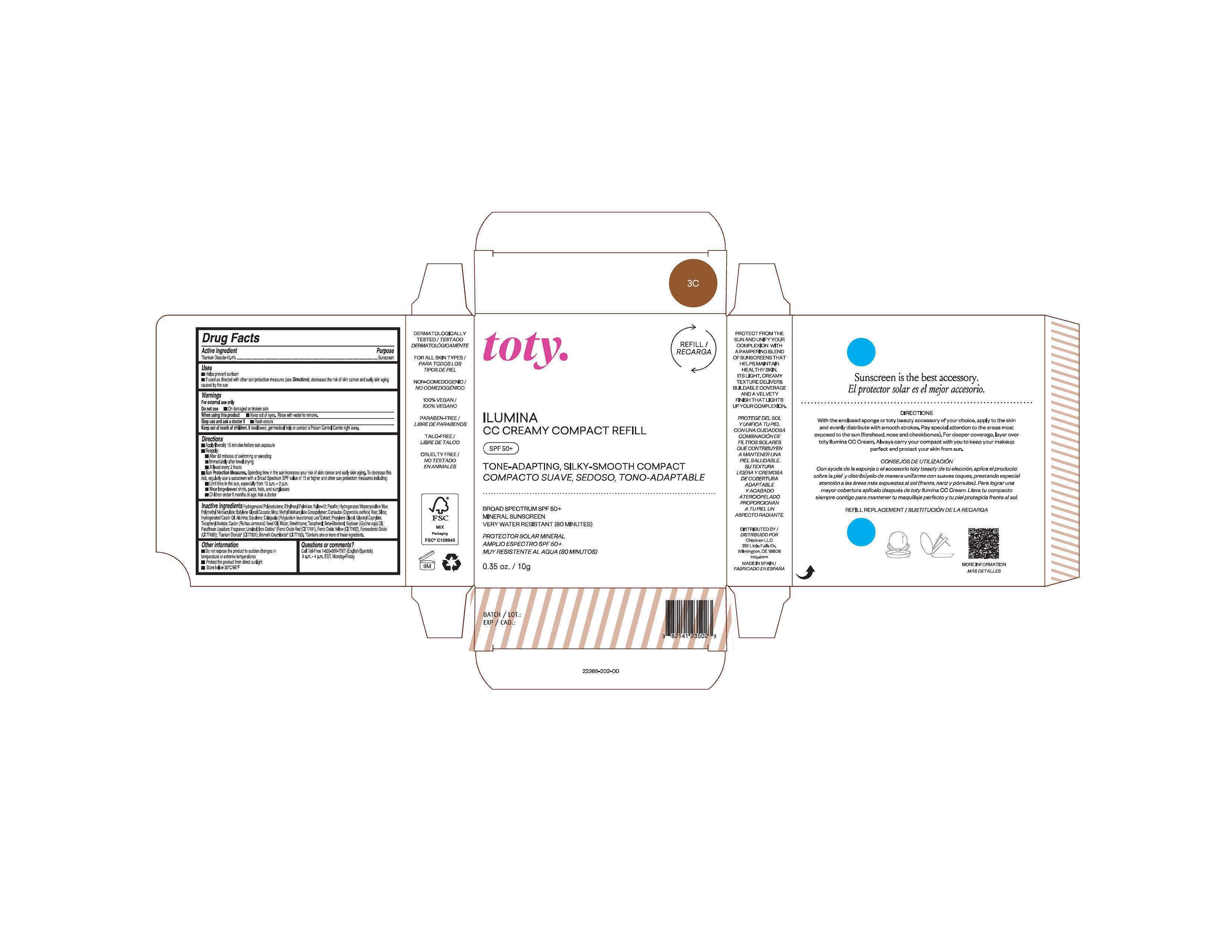
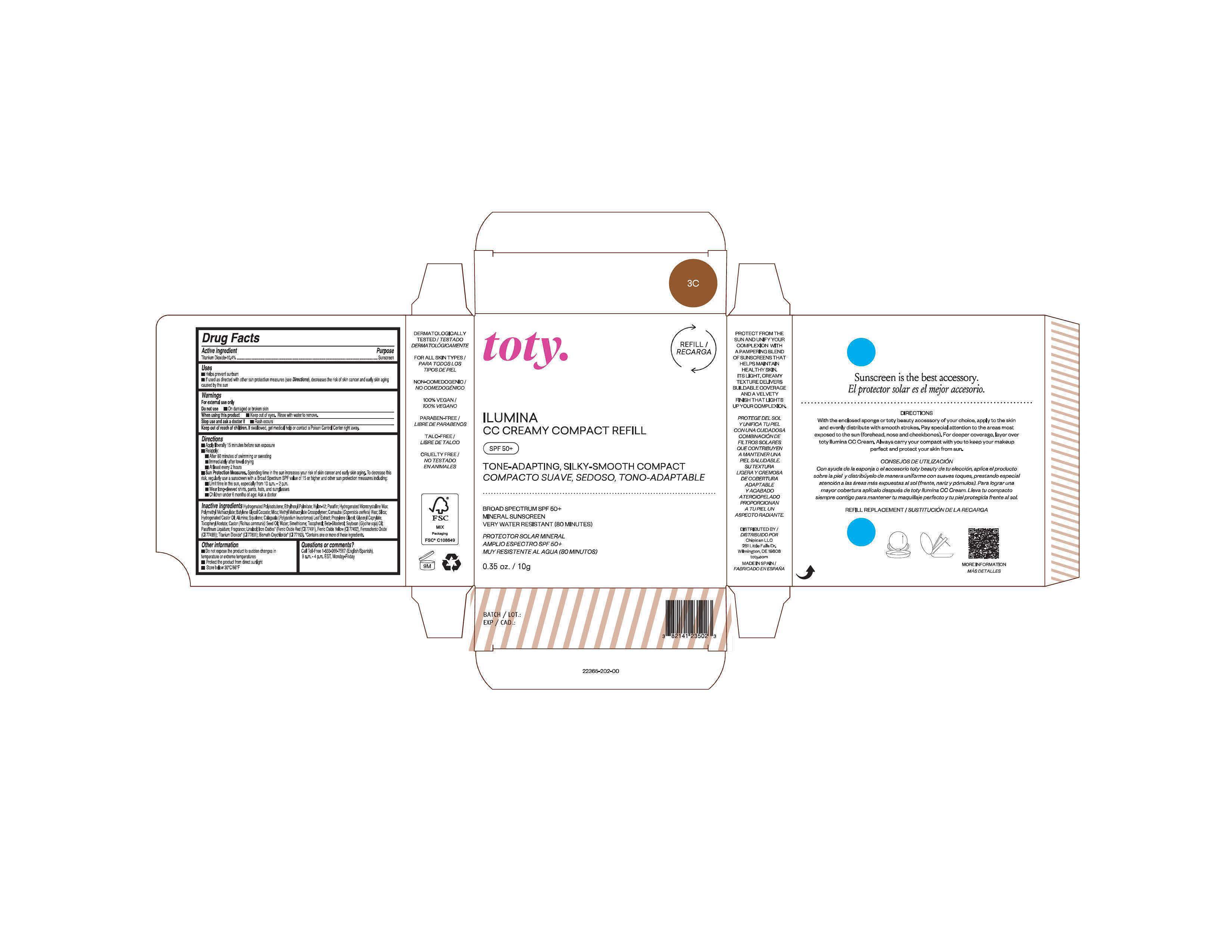
Package Label.Principal Display Panel-
3C
NDC 82141-2365-1 (toty Ilumina CC Creamy Compact Refill 3C)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
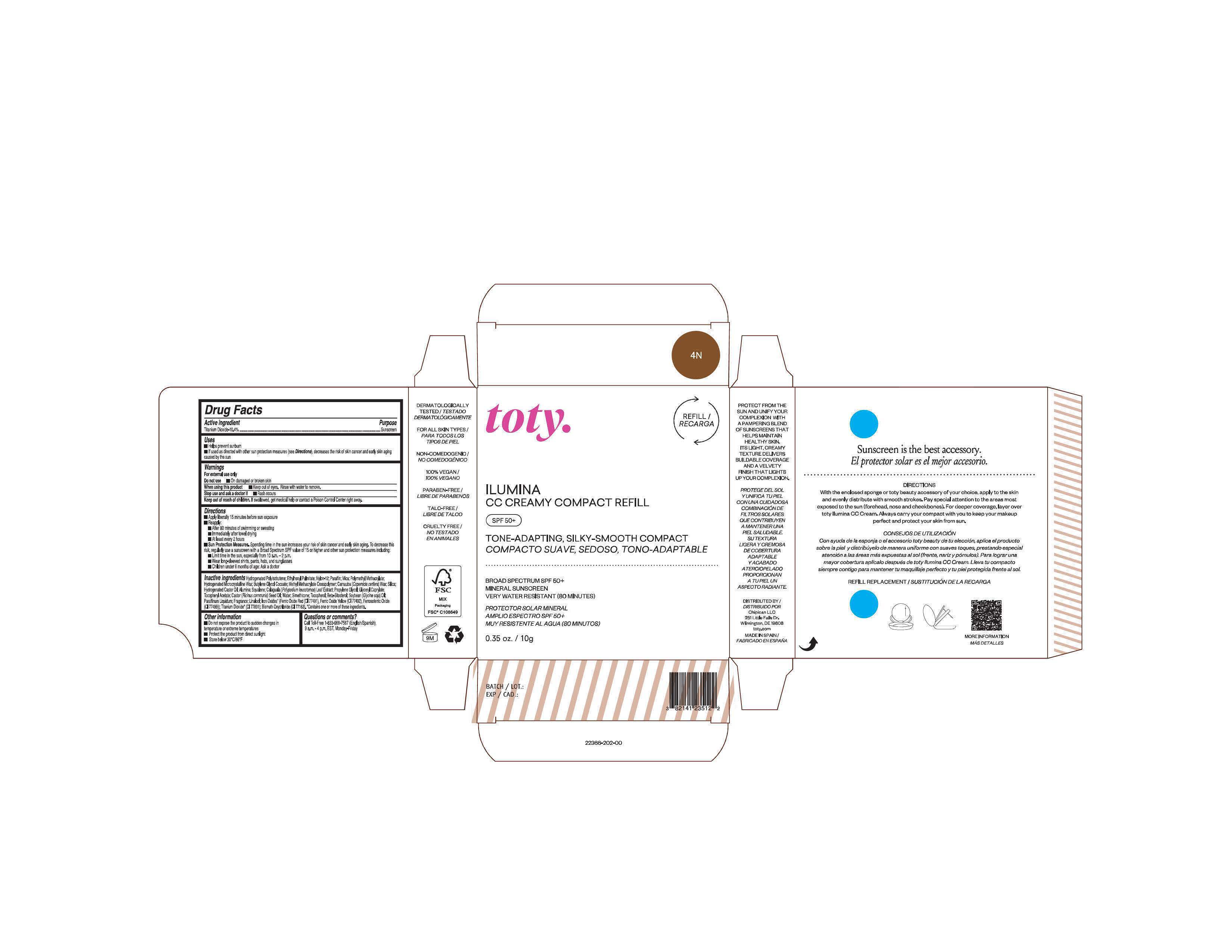
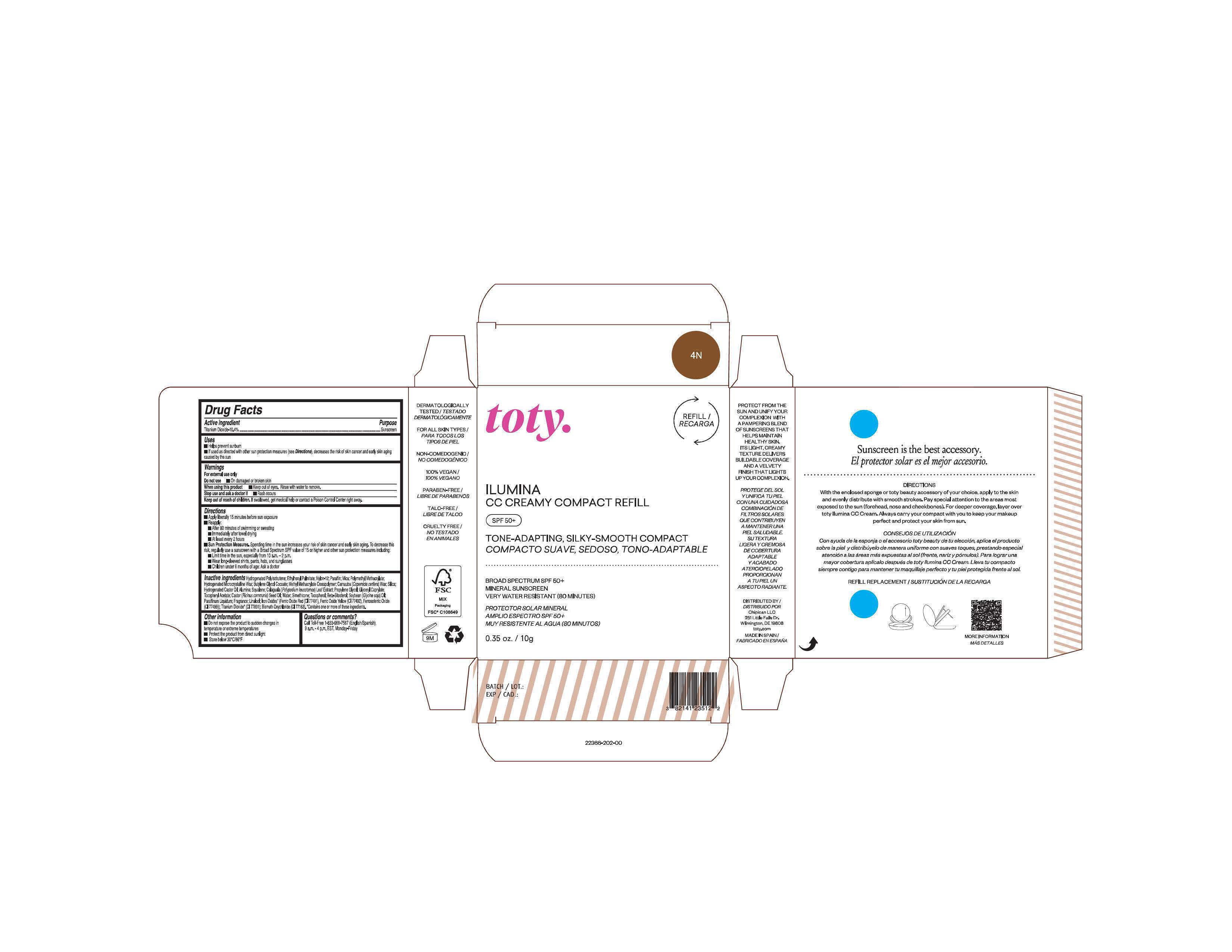
Package Label.Principal Display Panel-
4N
NDC 82141-2366-1 (toty Ilumina CC Creamy Compact Refill 4N)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
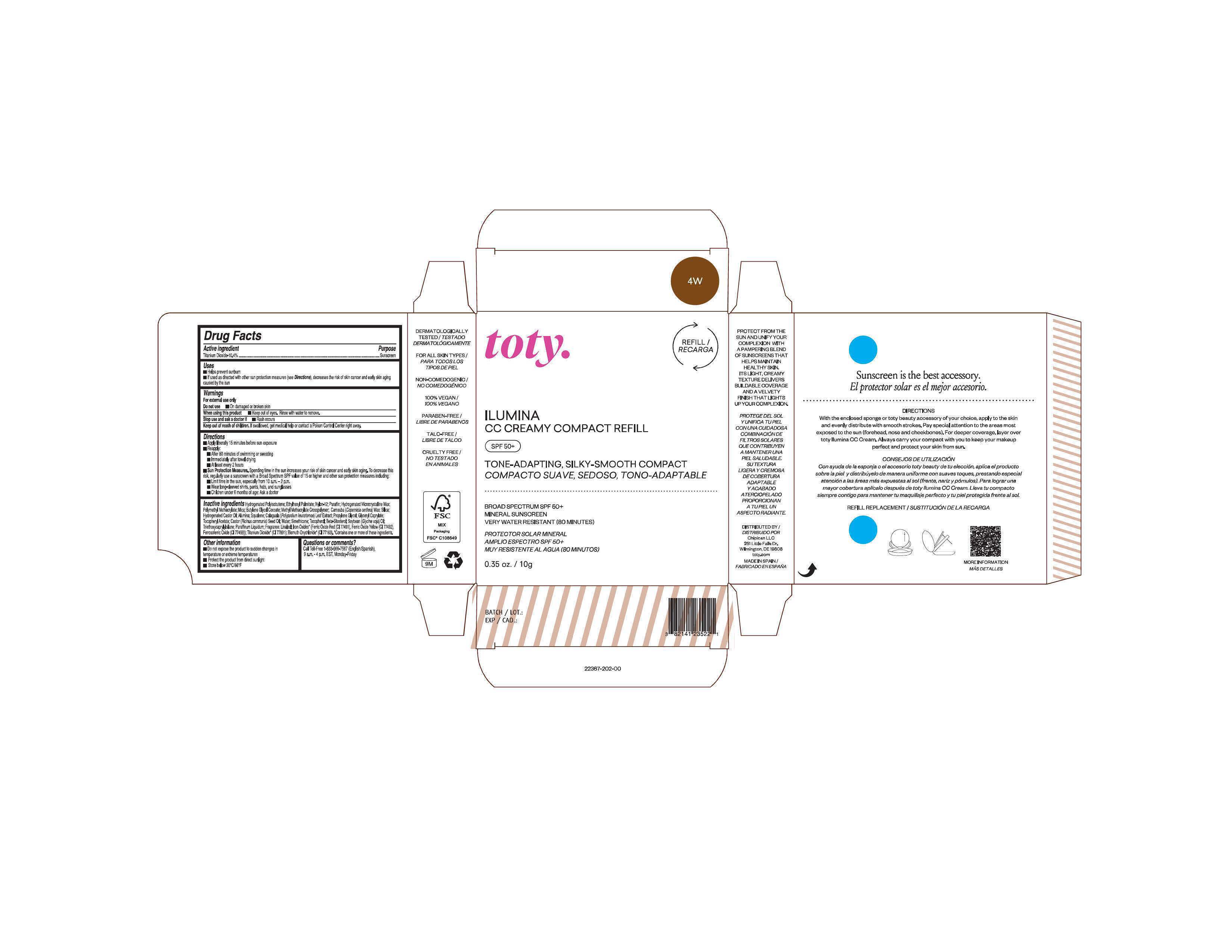
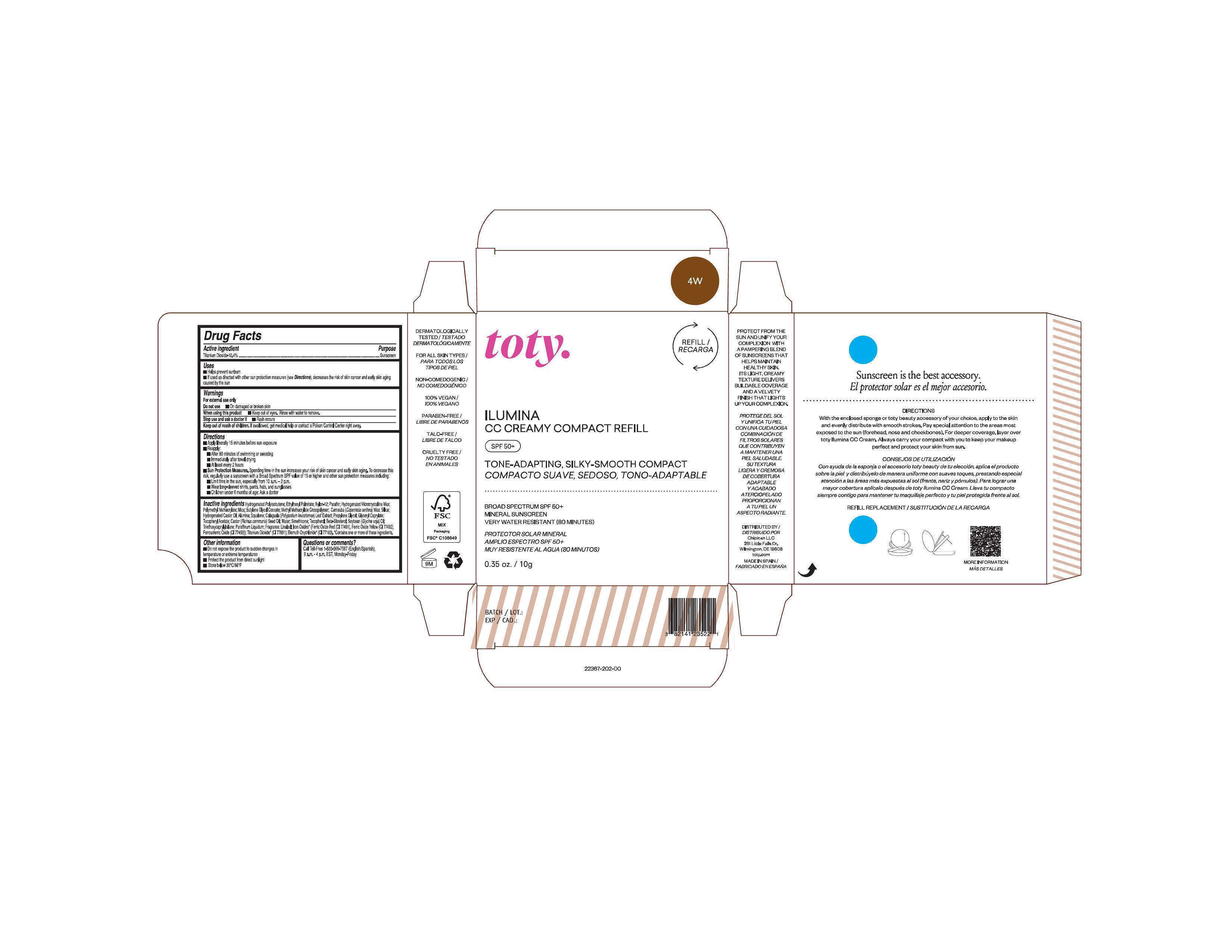
Package Label.Principal Display Panel-
4W
NDC 82141-2367-1 (toty Ilumina CC Creamy Compact Refill 4W)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g
-
Package Label.Principal Display Panel-
4C
NDC 82141-2368-1 (toty Ilumina CC Creamy Compact Refill 4C)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
Package Label.Principal Display Panel-
5W
NDC 82141-2369-1 (toty Ilumina CC Creamy Compact Refill 5W)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
Package Label.Principal Display Panel-
5W1
NDC 82141-2370-1 (toty Ilumina CC Creamy Compact Refill 5W1)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
Package Label.Principal Display Panel-
5W2
NDC 82141-2371-1 (toty Ilumina CC Creamy Compact Refill 5W2)
toty.
REFILL/RECARGA
ILUMINA
CC CREAMY COMPACT REFILL
SPF 50+
TONE-ADAPTING, SILKY-SMOOTH COMPACT
COMPACTO SUAVE, SEDOSO, TONO-ADAPTABLE
.....................................................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
VERY WATER RESISTANT (80 MINUTES)
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
0.35 oz/10g

-
INGREDIENTS AND APPEARANCE
TOTY ILUMINA CC CREAMY COMPACT REFILL 4C
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2368 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2368-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 3C
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2365 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2365-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 3W
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2364 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2364-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 4N
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2366 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2366-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 1C
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2359 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2359-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 2W
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2361 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2361-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 5W1
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2370 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2370-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 5W2
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2371 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2371-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 3N
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2363 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2363-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 1N
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2357 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2357-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 1W
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2358 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2358-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 2N
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2360-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 2C
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2362 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2362-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 4W
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2367 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2367-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 TOTY ILUMINA CC CREAMY COMPACT REFILL 5W
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2369 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10.4 g in 100 g Inactive Ingredients Ingredient Name Strength TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MINERAL OIL (UNII: T5L8T28FGP) 1,2-BUTYLENE GLYCOL MONOCOCOATE (UNII: XK3264896J) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) SOYBEAN OIL (UNII: 241ATL177A) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) WATER (UNII: 059QF0KO0R) .BETA.-SITOSTEROL (UNII: S347WMO6M4) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) NYLON-12 (UNII: 446U8J075B) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MICA (UNII: V8A1AW0880) SQUALENE (UNII: 7QWM220FJH) CASTOR OIL (UNII: D5340Y2I9G) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CARNAUBA WAX (UNII: R12CBM0EIZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2369-1 1 in 1 CARTON 05/31/2023 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/31/2023 Labeler - Chipican LLC (118132015) Establishment Name Address ID/FEI Business Operations Industrial Farmaceutica Cantabria SA 470471158 manufacture(82141-2357, 82141-2358, 82141-2359, 82141-2360, 82141-2361, 82141-2362, 82141-2363, 82141-2364, 82141-2365, 82141-2366, 82141-2367, 82141-2368, 82141-2369, 82141-2370, 82141-2371) , pack(82141-2357, 82141-2358, 82141-2359, 82141-2360, 82141-2361, 82141-2362, 82141-2363, 82141-2364, 82141-2365, 82141-2366, 82141-2367, 82141-2368, 82141-2369, 82141-2370, 82141-2371) , label(82141-2357, 82141-2358, 82141-2359, 82141-2360, 82141-2361, 82141-2362, 82141-2363, 82141-2364, 82141-2365, 82141-2366, 82141-2367, 82141-2368, 82141-2369, 82141-2370, 82141-2371)