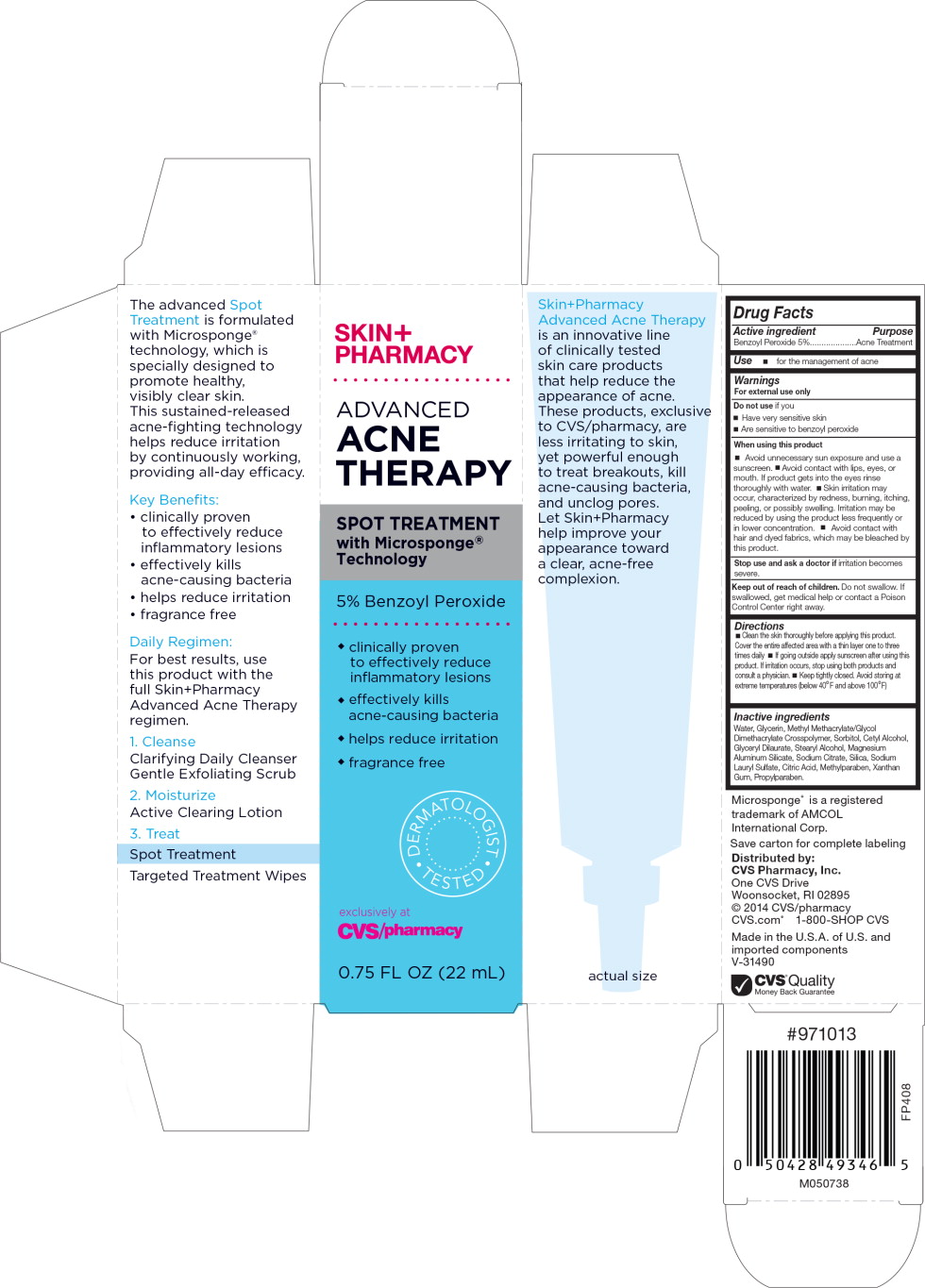
Label: SKINPHARMACY ADVANCED ACNE THERAPY SPOT TREATMENT- benzoyl peroxide liquid
- NDC Code(s): 69842-023-01
- Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- Avoid unnecessary sun exposure and use a sunscreen.
- Avoid contact with lips, eyes, or mouth. If product gets into the rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
-
Directions
- Clean skin thoroughly before applying this product. Cover the entire affected area with a thin layer one to three times daily.
- If going outside apply sunscreen after using this product. If irritation occurs, stop using both products and consult a physician.
- Keep tightly closed Avoid storing at extreme temperature (below 40°F and above 100°F)
-
Inactive ingredients
Water, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Sorbitol, Cetyl Alcohol, Glyceryl Dilaurate, Stearyl Alcohol, Magnesium Aluminum Silicate, Sodium Citrate, Silica, Sodium Lauryl Sulfate, Citric Acid, Methylparaben, Xanthan Gum, Propylparaben.
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and imported components
V-31490
CVS® Quality
Money Back Guarantee
#971013
M050738
FP408
-
Principal Display Panel - Carton Label
SKIN+
PHARMACYADVANCED
ACNE
THERAPYSPOT TREATMENT
with Microsponge® Technology5% Benzoyl Peroxide
- clinically proven
to effectively reduce
inflammatory lesions - effectively kills
acne-causing bacteria - helps reduce irritation
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy0.75 FL OZ (22 mL)
- clinically proven
-
Principal Display Panel - Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPYSPOT TREATMENT
with Microsponge® Technology5% Benzoyl Peroxide
- clinically proven
to effectively reduce
inflammatory lesions - effectively kills
acne-causing bacteria - helps reduce irritation
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy0.75 FL OZ (22 mL)
- clinically proven
-
INGREDIENTS AND APPEARANCE
SKINPHARMACY ADVANCED ACNE THERAPY SPOT TREATMENT
benzoyl peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength benzoyl peroxide (UNII: W9WZN9A0GM) (benzoyl peroxide - UNII:W9WZN9A0GM) benzoyl peroxide 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) glycerin (UNII: PDC6A3C0OX) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) cetyl alcohol (UNII: 936JST6JCN) sorbitol (UNII: 506T60A25R) glyceryl dilaurate (UNII: MFL3ZIE8SK) stearyl alcohol (UNII: 2KR89I4H1Y) magnesium aluminum silicate (UNII: 6M3P64V0NC) sodium citrate (UNII: 1Q73Q2JULR) silicon dioxide (UNII: ETJ7Z6XBU4) sodium lauryl sulfate (UNII: 368GB5141J) citric acid monohydrate (UNII: 2968PHW8QP) methylparaben (UNII: A2I8C7HI9T) xanthan gum (UNII: TTV12P4NEE) propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-023-01 1 in 1 CARTON 06/01/2014 1 22 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/01/2014 Labeler - CVS Health (062312574) Registrant - AMCOL Health & Beauty Solutions, Inc. DBA (872684803) Establishment Name Address ID/FEI Business Operations AMCOL Health & Beauty Solutions, Inc. DBA 872684803 MANUFACTURE(69842-023) , PACK(69842-023) , LABEL(69842-023) , ANALYSIS(69842-023)