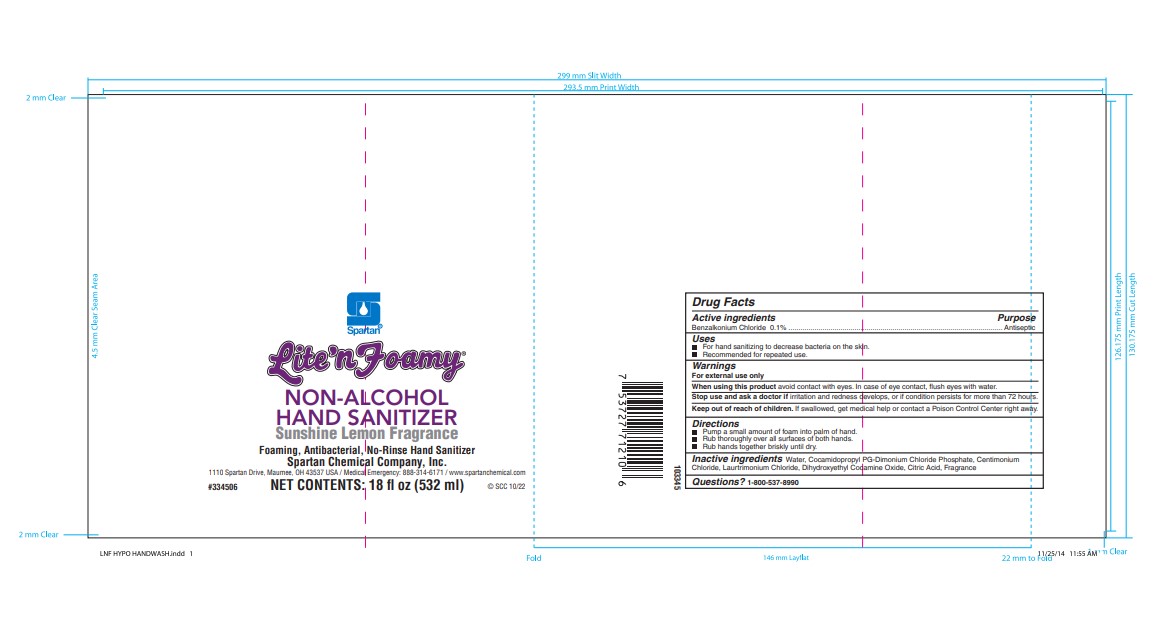
Label: LITE N FOAMY NON-ALCOHOL HAND SANITIZER soap
- NDC Code(s): 64009-222-82, 64009-222-85, 64009-222-88
- Packager: Spartan Chemical Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only.
When using this product avoid contact with eyes. In case of eye contact, flush eyes with water.
Stop use and ask a doctor if irritation and redness develops, if condition persists for more than 72 hours consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
- Warnings Section
- Directions
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LITE N FOAMY NON-ALCOHOL HAND SANITIZER
lite n foamy non-alcohol hand sanitizer soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64009-222 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) FRAGRANCE 13576 (UNII: 5EM498GW35) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIHYDROXYETHYL COCAMINE OXIDE (UNII: 8AR51R3BL5) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) LAURTRIMONIUM CHLORIDE (UNII: A81MSI0FIC) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64009-222-85 3790 g in 1 CONTAINER; Type 0: Not a Combination Product 12/31/2022 2 NDC:64009-222-88 208200 g in 1 CONTAINER; Type 0: Not a Combination Product 12/31/2022 3 NDC:64009-222-82 532 g in 1 CONTAINER; Type 0: Not a Combination Product 12/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/31/2022 Labeler - Spartan Chemical Company (005036728) Establishment Name Address ID/FEI Business Operations Spartan Chemical Company 005036728 manufacture(64009-222)