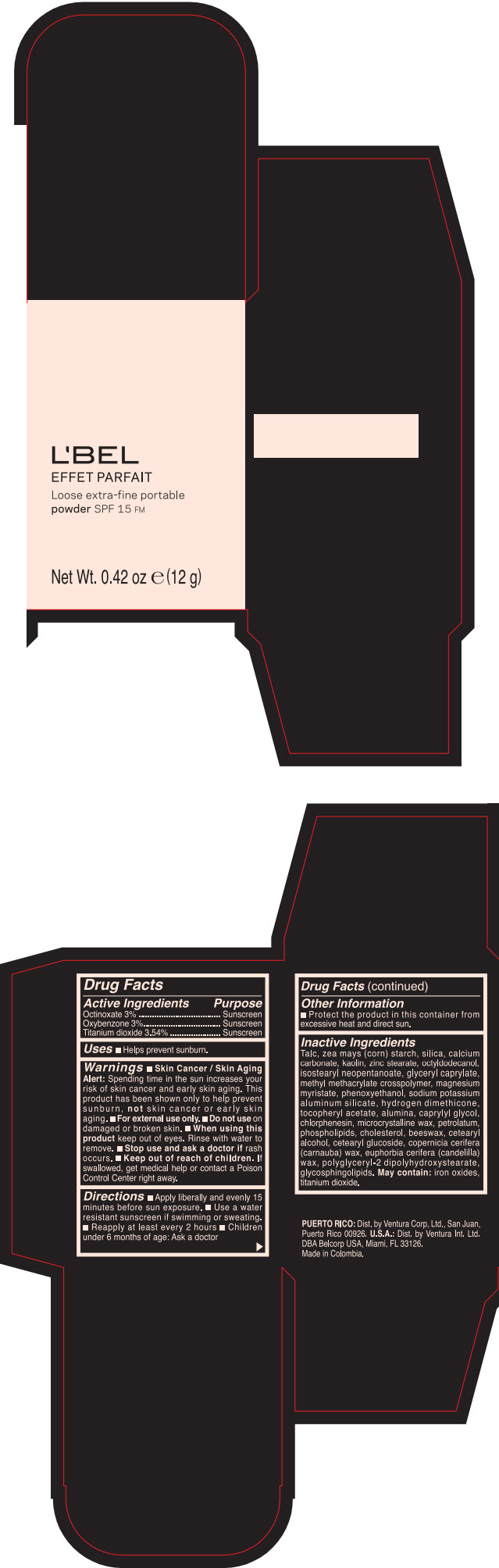
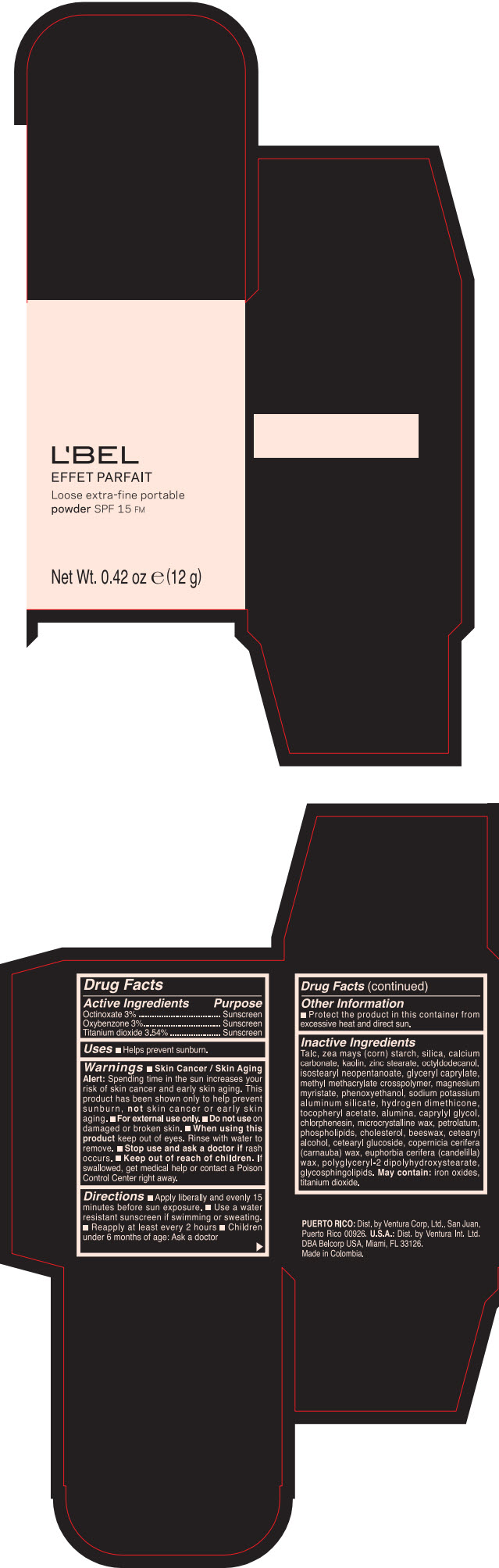
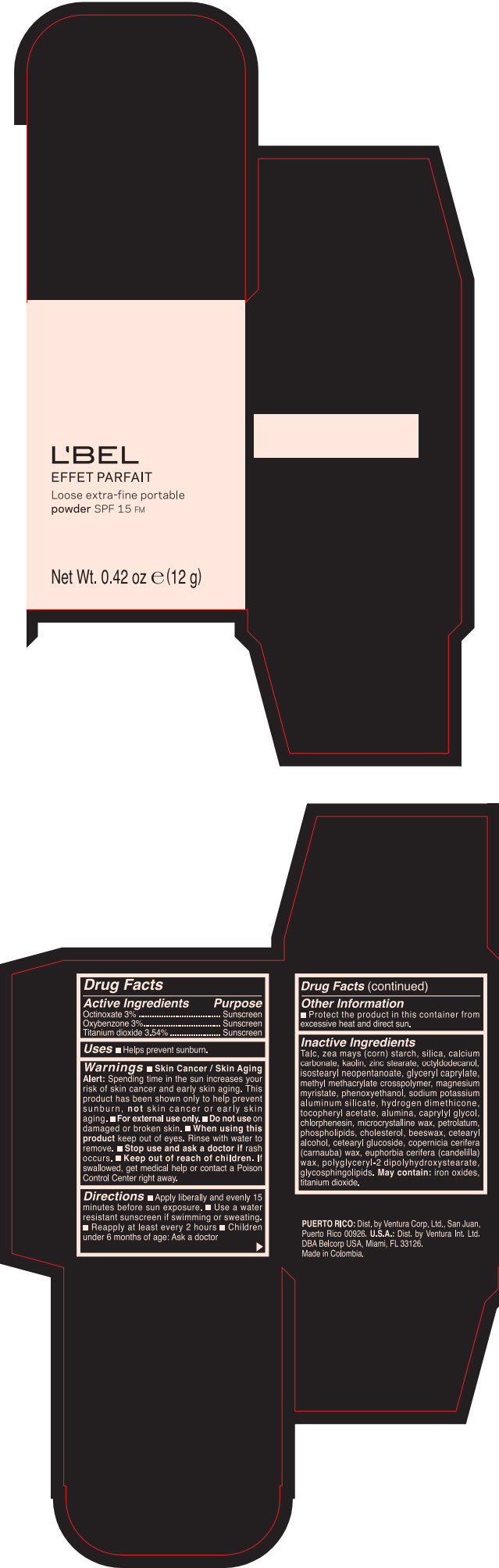
Label: LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM PORCELAINE- BEIGE- octinoxate, titanium dioxide, and oxybenzone powder
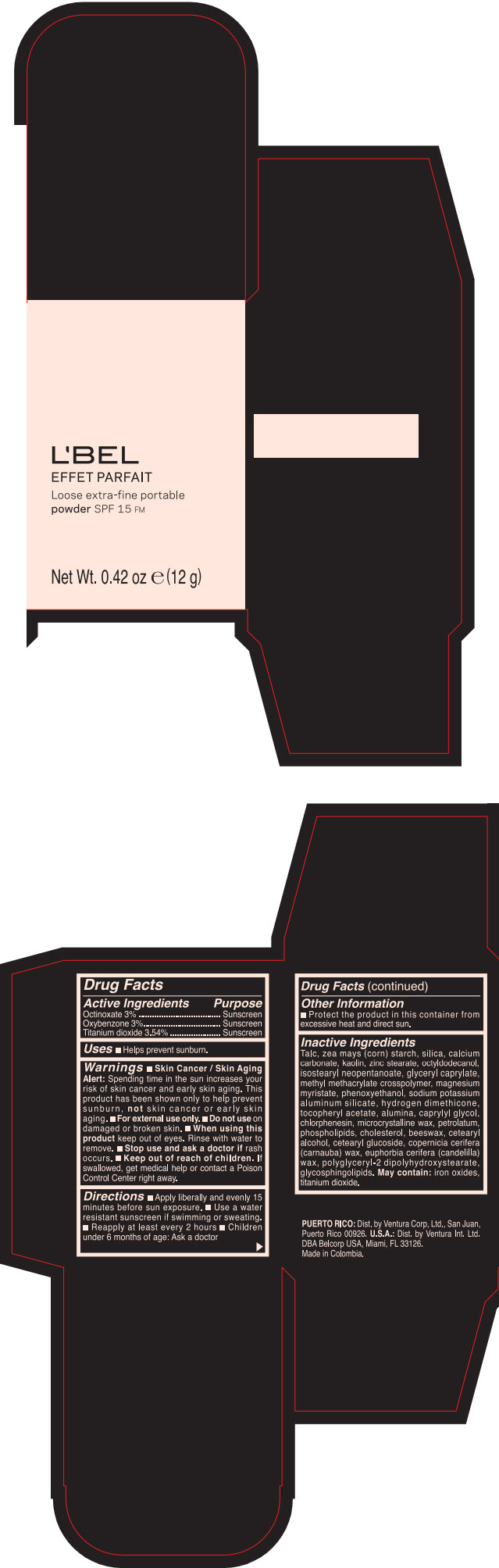
LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM TRANSLUCIDE- BEIGE- octinoxate, titanium dioxide, and oxybenzone powder
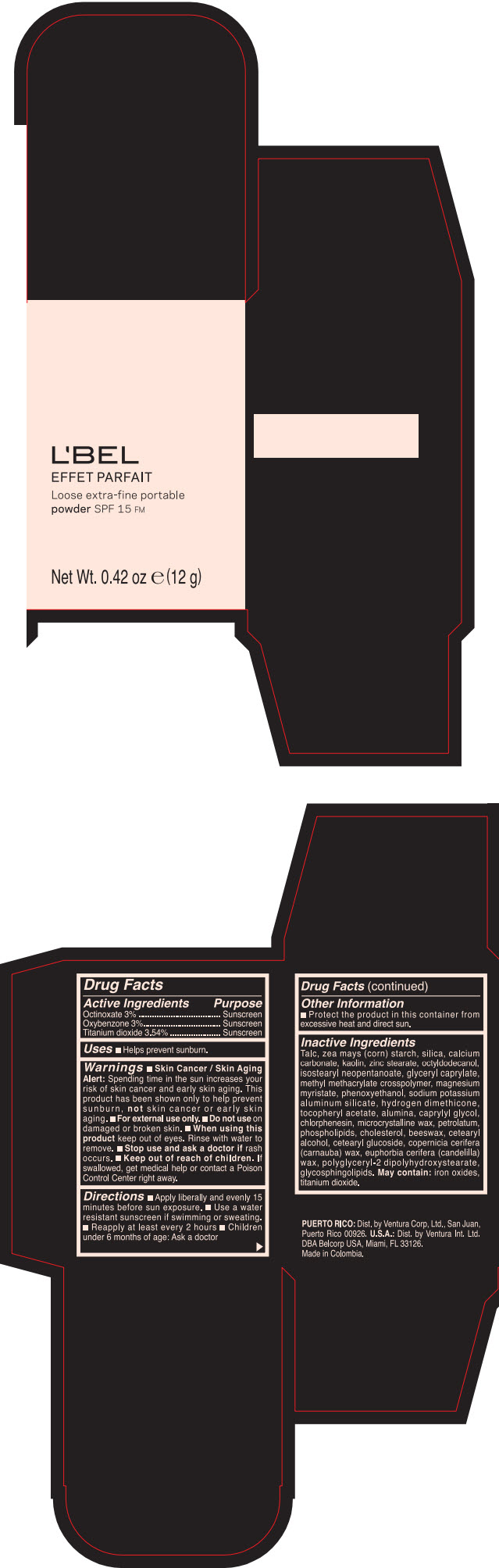
LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM SANTE- BEIGE- octinoxate, titanium dioxide, and oxybenzone powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 14783-721-01, 14783-721-02, 14783-722-01, 14783-722-02, view more14783-723-01, 14783-723-02 - Packager: Ventura International LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
- Skin Cancer / Skin Aging Alert; Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
- Do not use on damage or broken skin.
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Directions
- Other information
-
Inactive ingredients
TALC, ZEA MAYS (CORN) STARCH, SILICA, CALCIUM CARBONATE, KAOLIN, ZINC STEARATE, OCTYLDODECANOL, ISOSTEARYL NEOPENTANOATE, GLYCERYL CAPRYLATE, METHYL METHACRYLATE CROSSPOLYMER, MAGNESIUM MYRISTATE, PHENOXYETHANOL, SODIUM POTASSIUM ALUMINUM SILICATE, HYDROGEN DIMETHICONE, TOCOPHERYL ACETATE, ALUMINA, CAPRYLYL GLYCOL, CHLORPHENESIN, MICROCRYSTALLINE WAX, PETROLATUM, PHOSPHOLIPIDS, CHOLESTEROL, EUPHORBIA CERIFERA (CANDELILLA) WAX, BEESWAX, CETEARYL ALCOHOL, CETEARYL GLUCOSIDE, COPERNICIA CERIFERA (CARNAUBA) WAX, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, GLYCOSPHINGOLIPIDS.
MAY CONTAIN:
IRON OXIDES, TITANIUM DIOXIDE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 12 g Case Box - PORCELAINE - BEIGE
- PRINCIPAL DISPLAY PANEL - 12 g Case Box - TRANSLUCIDE- BEIGE
- PRINCIPAL DISPLAY PANEL - 12 g Case Box - SANTE- BEIGE
-
INGREDIENTS AND APPEARANCE
LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM PORCELAINE- BEIGE
octinoxate, titanium dioxide, and oxybenzone powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-721 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.03 g in 1 g Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.0354 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.03 g in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM CARBONATE (UNII: H0G9379FGK) KAOLIN (UNII: 24H4NWX5CO) ZINC STEARATE (UNII: H92E6QA4FV) OCTYLDODECANOL (UNII: 461N1O614Y) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALUMINUM OXIDE (UNII: LMI26O6933) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CHOLESTEROL (UNII: 97C5T2UQ7J) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-721-02 1 in 1 BOX 12/22/2015 1 NDC:14783-721-01 12 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/22/2015 LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM TRANSLUCIDE- BEIGE
octinoxate, titanium dioxide, and oxybenzone powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-722 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.03 g in 1 g Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.0354 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.03 g in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM CARBONATE (UNII: H0G9379FGK) KAOLIN (UNII: 24H4NWX5CO) ZINC STEARATE (UNII: H92E6QA4FV) OCTYLDODECANOL (UNII: 461N1O614Y) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALUMINUM OXIDE (UNII: LMI26O6933) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CHOLESTEROL (UNII: 97C5T2UQ7J) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-722-02 1 in 1 BOX 12/22/2015 1 NDC:14783-722-01 12 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/22/2015 LBEL EFFET PARFAIT LOOSE EXTRA-FINE PORTABLE SPF 15 FM SANTE- BEIGE
octinoxate, titanium dioxide, and oxybenzone powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-723 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.03 g in 1 g Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.0354 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.03 g in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CALCIUM CARBONATE (UNII: H0G9379FGK) KAOLIN (UNII: 24H4NWX5CO) ZINC STEARATE (UNII: H92E6QA4FV) OCTYLDODECANOL (UNII: 461N1O614Y) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALUMINUM OXIDE (UNII: LMI26O6933) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CHOLESTEROL (UNII: 97C5T2UQ7J) CANDELILLA WAX (UNII: WL0328HX19) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CARNAUBA WAX (UNII: R12CBM0EIZ) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-723-02 1 in 1 BOX 12/22/2015 1 NDC:14783-723-01 12 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/22/2015 Labeler - Ventura International LTD (603192787) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(14783-721, 14783-722, 14783-723)