Label: NIGHTTIME SLEEP-AID NON-HABIT FORMING- diphenhydramine hci liquid
- NDC Code(s): 76281-522-24, 76281-522-25, 76281-522-28
- Packager: AptaPharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- ASK DOCTOR/PHARMACIST
- When using this product
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
-
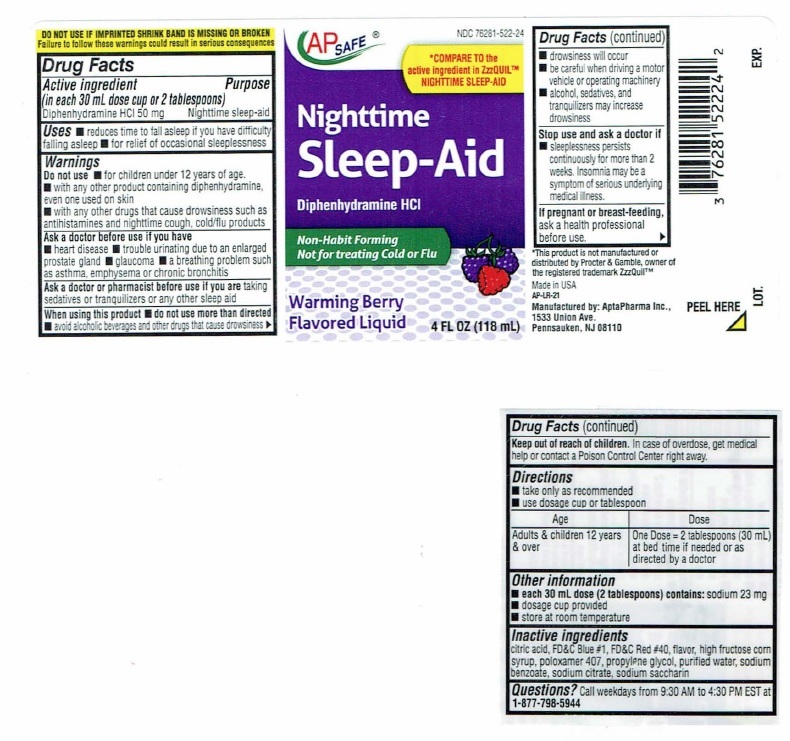
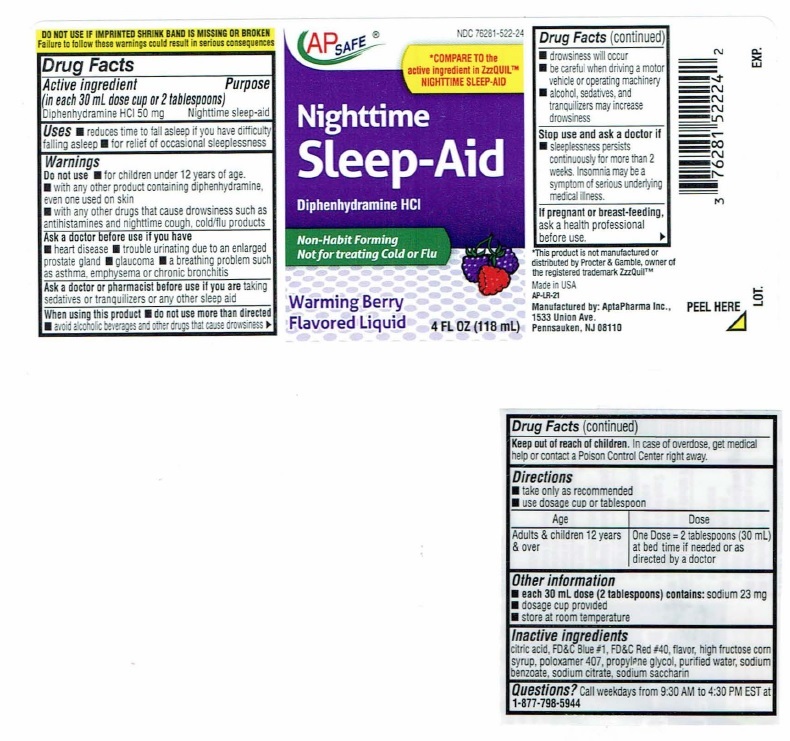
Nighttime Sleep-Aid Label
NDC 76281-522-28
AP SAFE
*COMPARE TO the
active ingredient in ZzzQUIL™
NIGHTTIME SLEEP-AIDNighttime
Sleep-AidDiphenhydramine HCI
Non-Habit Forming
Not for treating Cold or FluWarming Berry
Flavored Liquid12 FL OZ (354 mL)
DO NOTUSE IF IMPRINTEDSHRINK BAND IS MISSING OR BROKEN
Failure to follow these warnings could result in serious consequencesDrug Facts
Active ingredient Purpose
(in each 30 mL dose cup or 2 tablespoons)
Diphenhydramine HCI 50 mg…………………………Nighttime sleep-aidUses
■ reduces time to fall asleep if you have difficulty falling asleep
■ for relief of occasional sleeplessnessWarnings
Do not use ■ for children under 12 years of age.
■ with any other product containing diphenhydramine, even one
used on skin
■ with any other drugs that cause drowsiness such as
antihistamines and nighttime cough, cold/flu productsAsk a doctor before use if you have
■ heart disease
■ trouble urinating due to enlarged prostrate gland
■ glaucoma
■ a breathing problem such as asthma, emphysema or chronic
bronchitisAsk a doctor or pharmacist before use if you are taking
sedatives or tranquilizers or any other sleep aidWhen using this product
■ do not use more than directed
■ avoid alcoholic beverages and other drugs that cause drowsiness
■ drowsiness will occur
■ be careful when driving a motor vehicle or operating machinery
■ alcohol, sedatives, and tranquilizers may increase drowsinessStop use and ask a doctor if
■ sleeplessness persists continuously for more than 2 weeks.
Insomnia may be a symptom of serious underlying medical illness.If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help
or contact a Poison Control Center right away. ►Drug Facts(continued)
Directions
■ take only as recommended
■ Use dosage cup or tablespoonAge Dose
Adults & children 12 One Dose = 2 tablespoons (30 mL) at bed
years & over time if needed or as directed by a doctorOther information
■ each 30 mL dose (2 tablespoons) contains: sodium 23 mg
■ dosage cup provided
■ store at room temperatureInactive ingredients
citric acid, FD&C Blue #1, FD&C Red #40, flavor, high fructose corn
syrup, poloxamer 407, propylene glycol, purified water, sodium
benzoate, sodium citrate, sodium saccharinQuestions? Call weekdays from 9:30 AM to 4:30 PM EST at
1-877-798-5944*This product is not manufactured or distributed by Procter
& Gamble, owner of the registered trademark ZzzQuil™Manufactured by: AptaPharma Inc.,
1533 Union Ave.,
Pennsauken, NJ 08110AP-LR-10


6 OZ


4 OZ

res
-
INGREDIENTS AND APPEARANCE
NIGHTTIME SLEEP-AID NON-HABIT FORMING
diphenhydramine hci liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76281-522 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76281-522-28 354 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 2 NDC:76281-522-25 177 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 3 NDC:76281-522-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2018 Labeler - AptaPharma Inc. (790523323) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(76281-522)