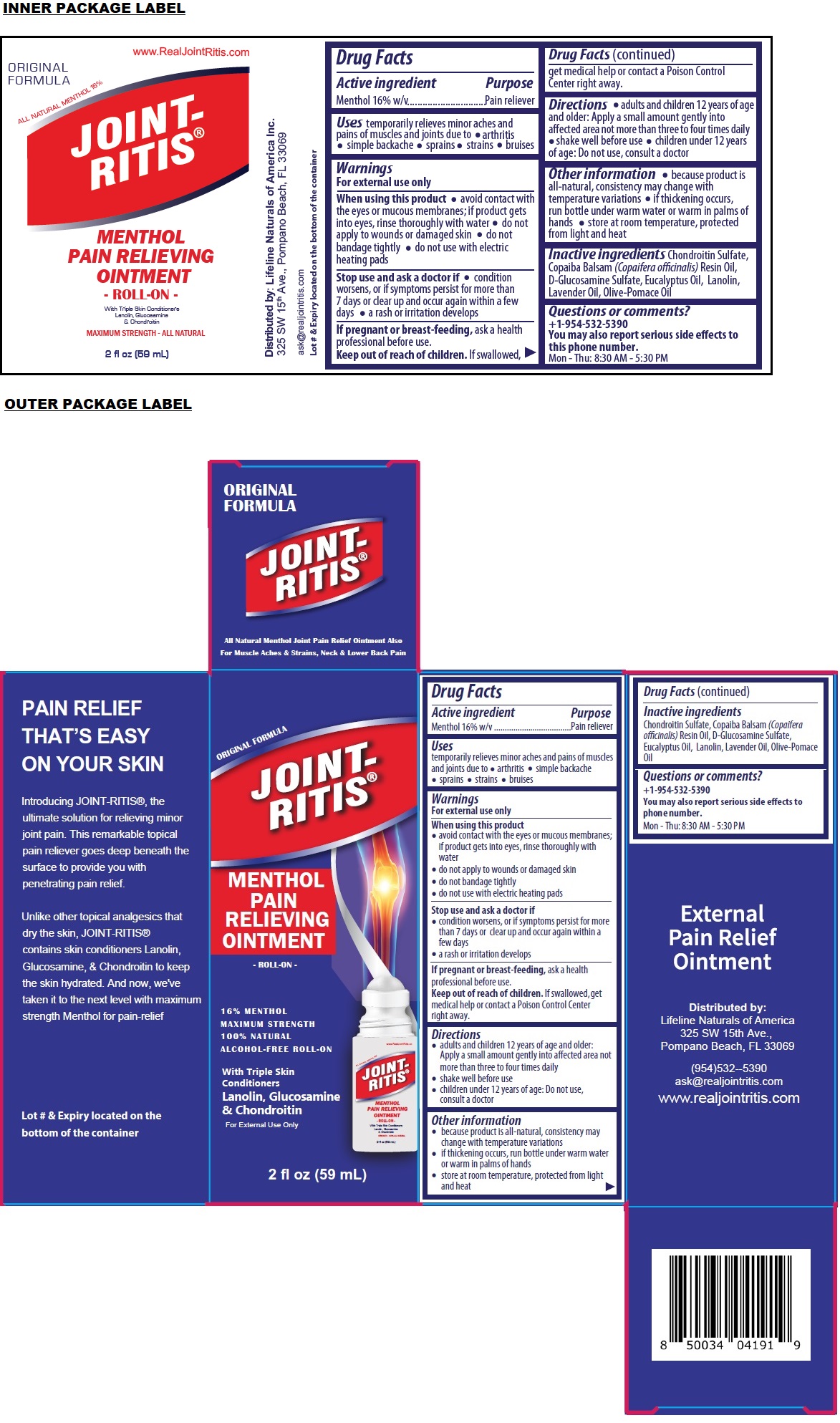
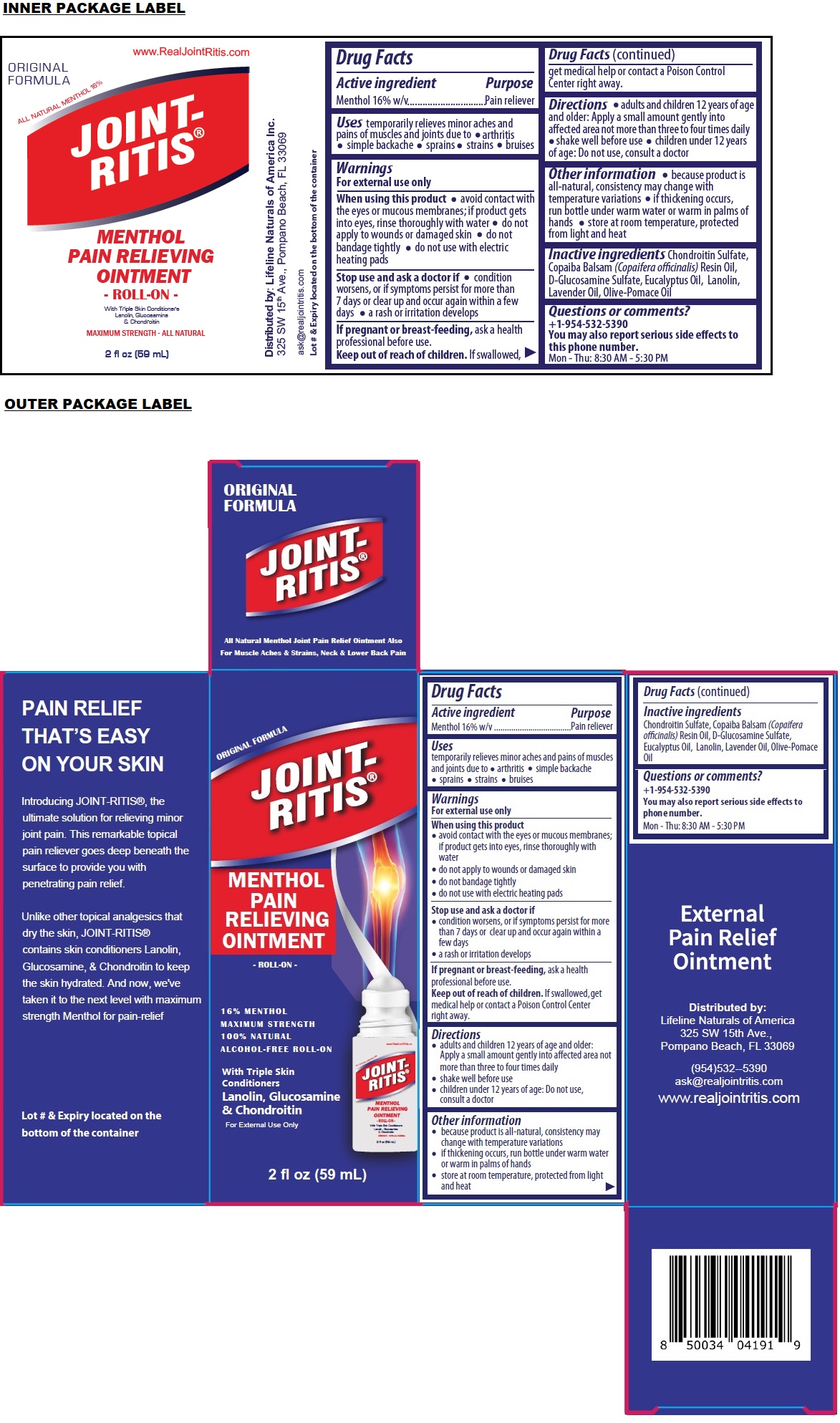
Label: JOINT-RITIS PAIN RELIEVING ROLL-ON- menthol ointment
- NDC Code(s): 83623-161-02, 83623-161-22
- Packager: Lifeline Naturals of America, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
• avoid contact with the eyes or mucous membranes; if product gets into eyes, rinse thoroughly with water
• do not apply to wounds or damaged skin
• do not bandage tightly
• do not use with electric heating padsStop use and ask a doctor if
• condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days
• a rash or irritation developsIf pregnant or breast-feeding, ask a health professional before use.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
ORIGINAL FORMULA
MAXIMUM STRENGTH
100% NATURAL
ALCOHOL-FREE ROLL-ONDistributed by:
Lifeline Naturals of America
325 SW 15th Ave.,
Pompano Beach, FL 33069
(954)532--5390
ask@realjointritis.com
www.realjointritis.comPAIN RELIEF THAT’S EASY ON YOUR SKIN
Introducing JOINT-RITIS®, the ultimate solution for relieving minor joint pain. This remarkable topical pain reliever goes deep beneath the surface to provide you with penetrating pain relief.
Unlike other topical analgesics that dry the skin, JOINT-RITIS® contains skin conditioners Lanolin, Glucosamine, & Chondroitin to keep the skin hydrated. And now, we've taken it to the next level with maximum strength Menthol for pain-relief
Lot # & Expiry located on the bottom of the container
All Natural Menthol Joint Pain Relief Ointment Also For Muscle Aches & Strains, Neck & Lower Back Pain
- Packaging
-
INGREDIENTS AND APPEARANCE
JOINT-RITIS PAIN RELIEVING ROLL-ON
menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83623-161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 16 g in 100 mL Inactive Ingredients Ingredient Name Strength CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) COPAIBA OIL (UNII: 64VX45Y68N) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) EUCALYPTUS OIL (UNII: 2R04ONI662) LANOLIN (UNII: 7EV65EAW6H) LAVENDER OIL (UNII: ZBP1YXW0H8) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83623-161-02 1 in 1 BOX 09/01/2023 1 NDC:83623-161-22 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M017 09/01/2023 Labeler - Lifeline Naturals of America, Inc. (056248953)