Label: WELLY CHILDRENS TRAVEL MEDICINE- acetaminophen, calcium carbonate, diphenhydramine hydrochloride kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 72663-203-49, 72663-225-49, 72663-233-49, 72663-347-49 - Packager: Welly Health PBC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 15, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
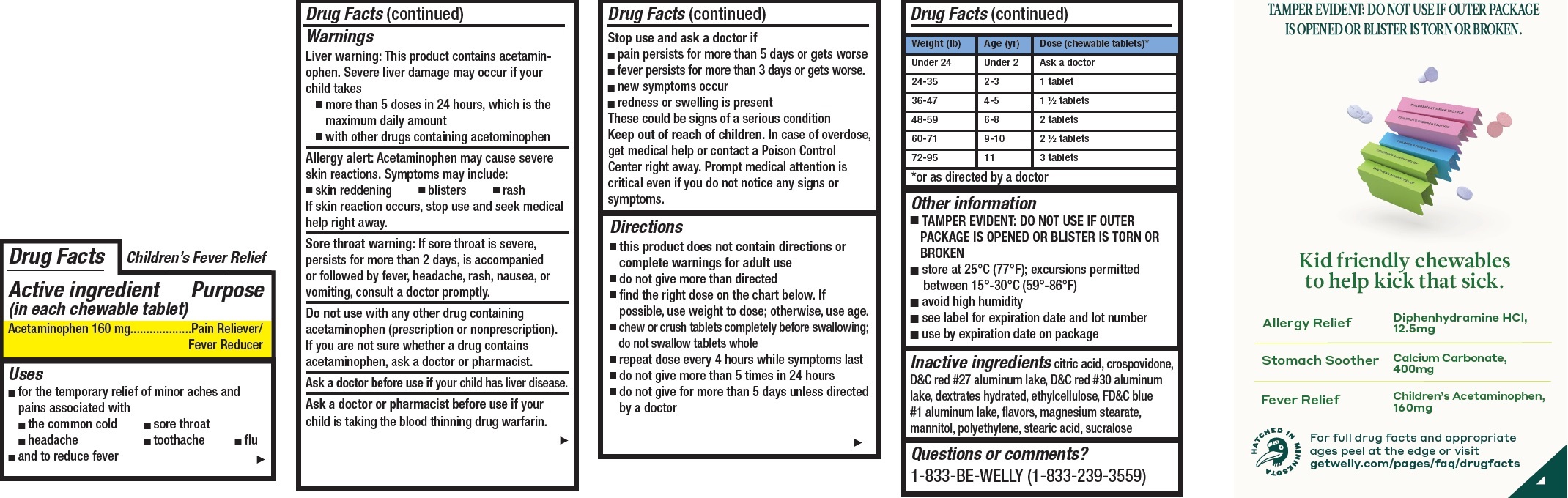
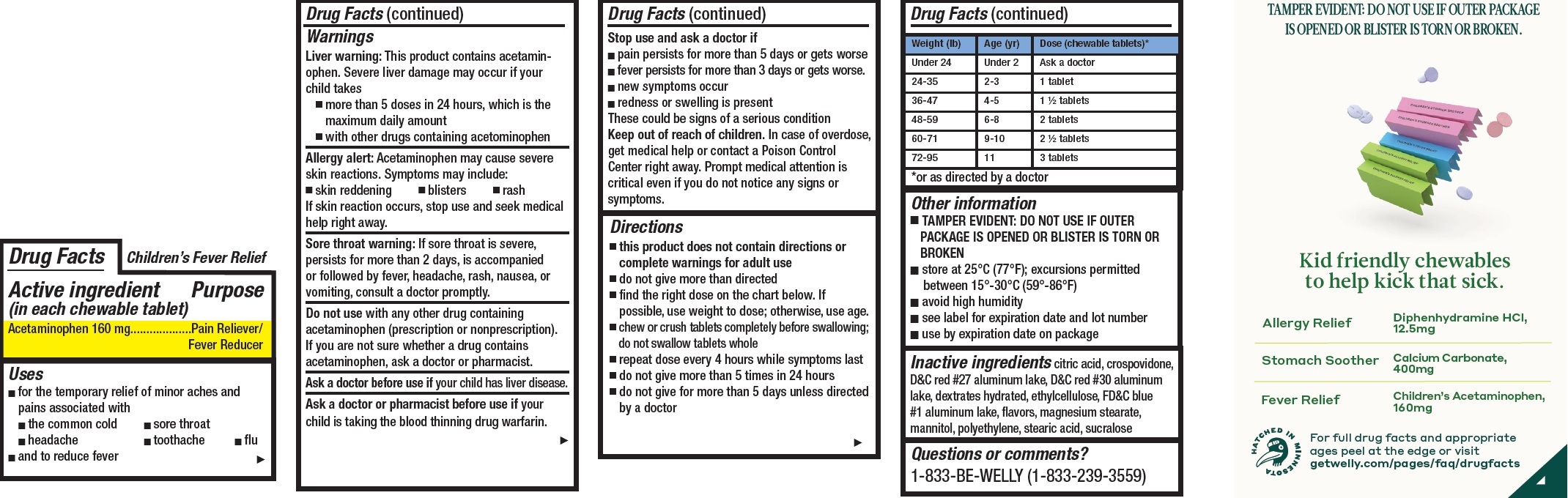
- Children's Pain and Fever Relief, 12 tablets
- Drug Facts
- Active ingredient (in each chewable tablet)
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetominophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on the chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
Weight (lb) Age (yr) Dose (chewable tablets)* Under 24 Under 2 Ask a doctor 24-35 2-3 1 tablet 36-47 4-5 1 1/2 tablets 48-59 6-8 2 tablets 60-71 9-10 2 1/2 tablets 72-95 11 3 tablets *or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
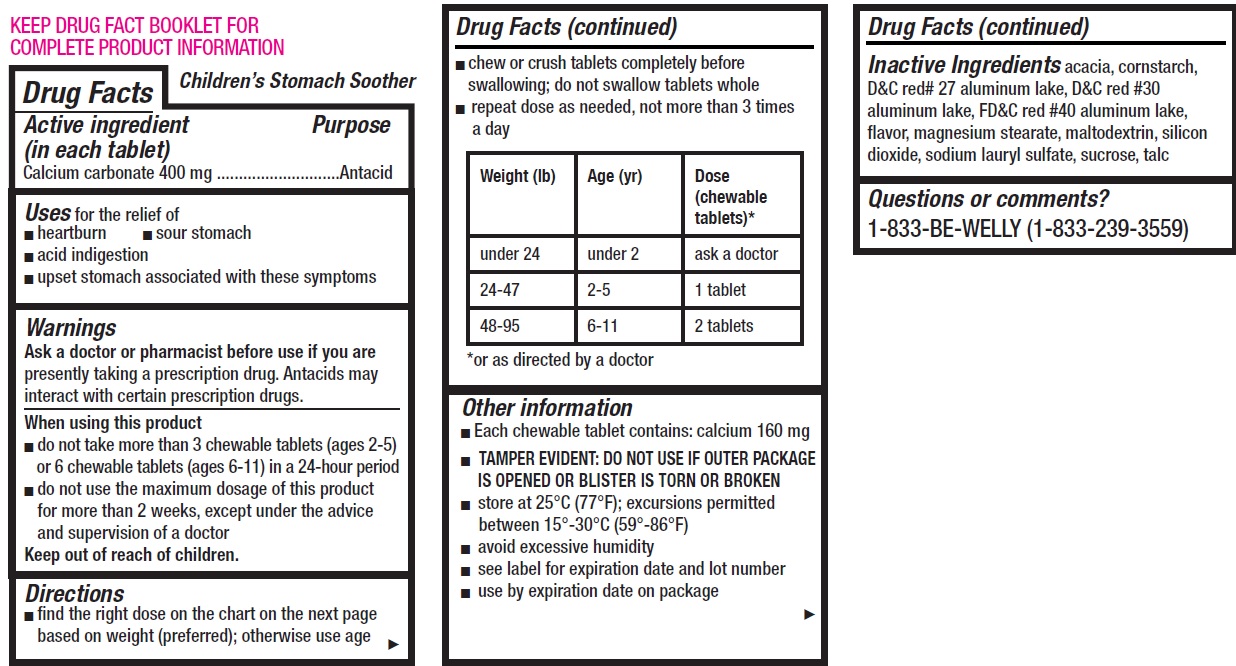
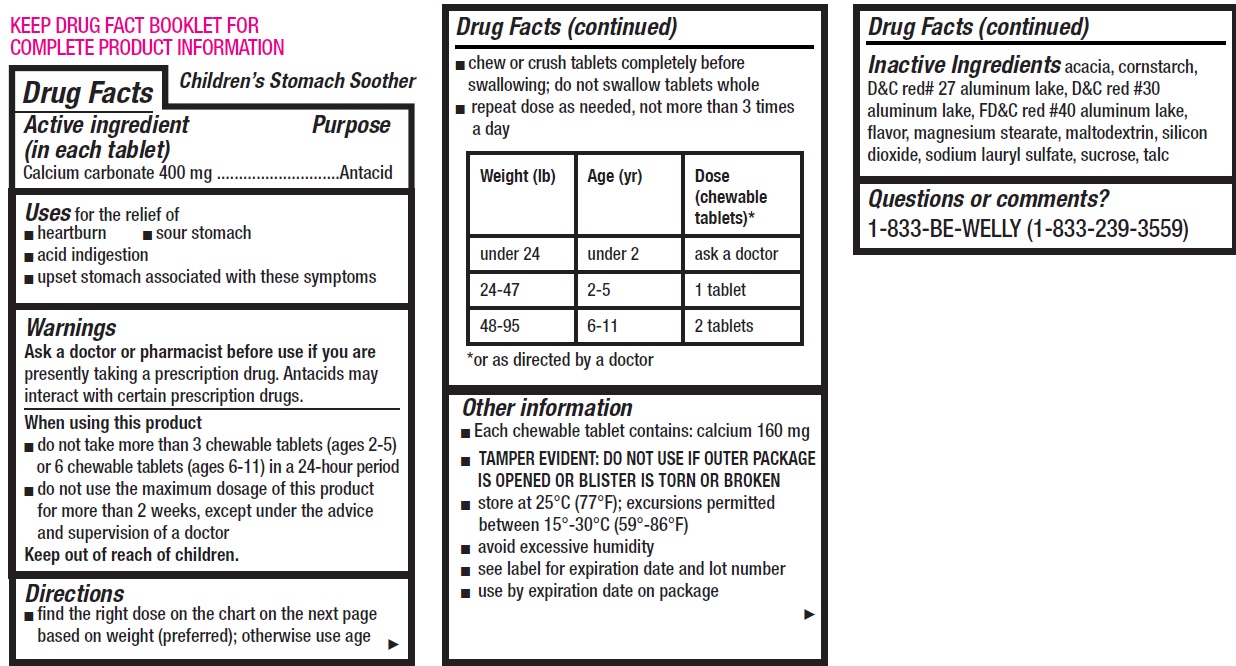
- Children's Stomach Soother, 12 tablets
- Drug Facts
- Active ingredient (in each tablet)
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
-
Directions
- find the right dose on the chart on the next page based on weight (preferred); otherwise use age
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose as needed, not more than 3 times a day
Weight (lb) Age (yr) Dose (chewable tablets)* under 24 under 2 ask a doctor 24-47 2-5 1 tablet 48-95 6-11 2 tablets *or as directed by a doctor
-
Other information
- Each chewable tablet contains: calcium 160 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- avoid excessive humidity
- see label for expiration date and lot number
- use by expiration date on package
- Inactive Ingredients
- Questions or comments?
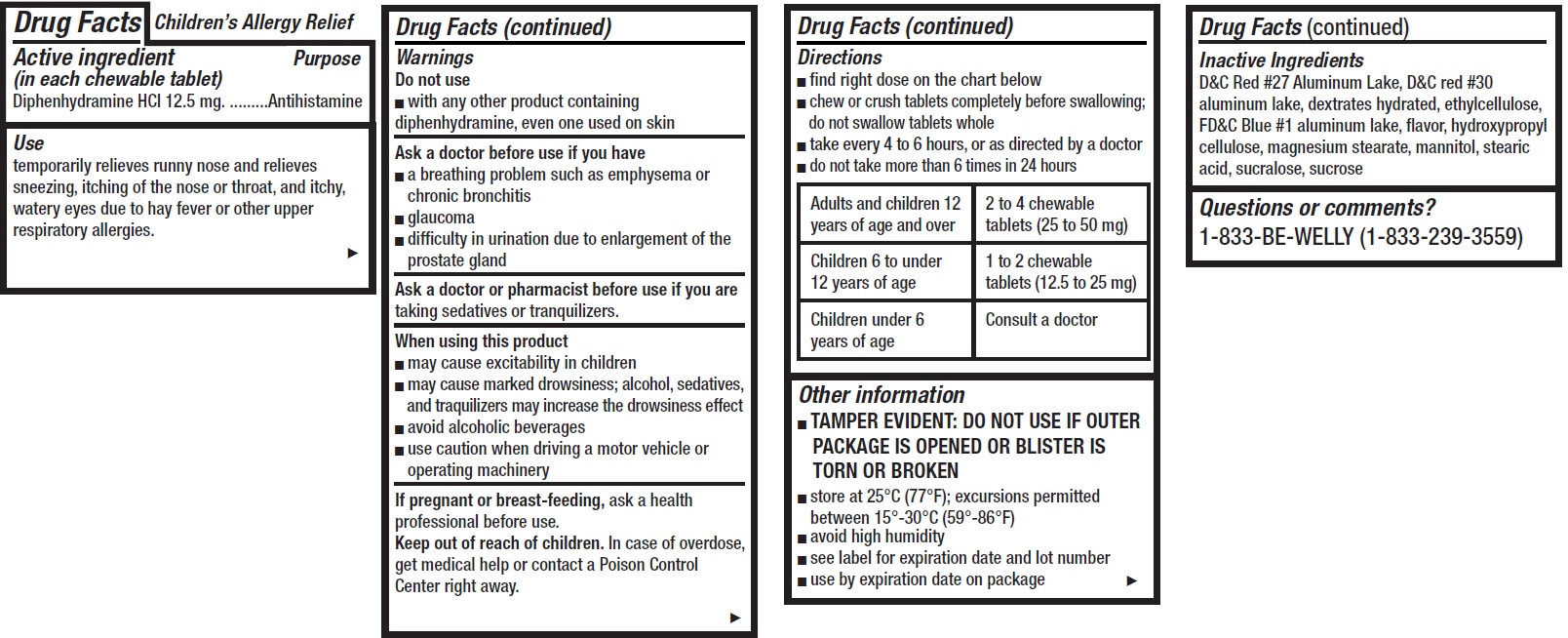
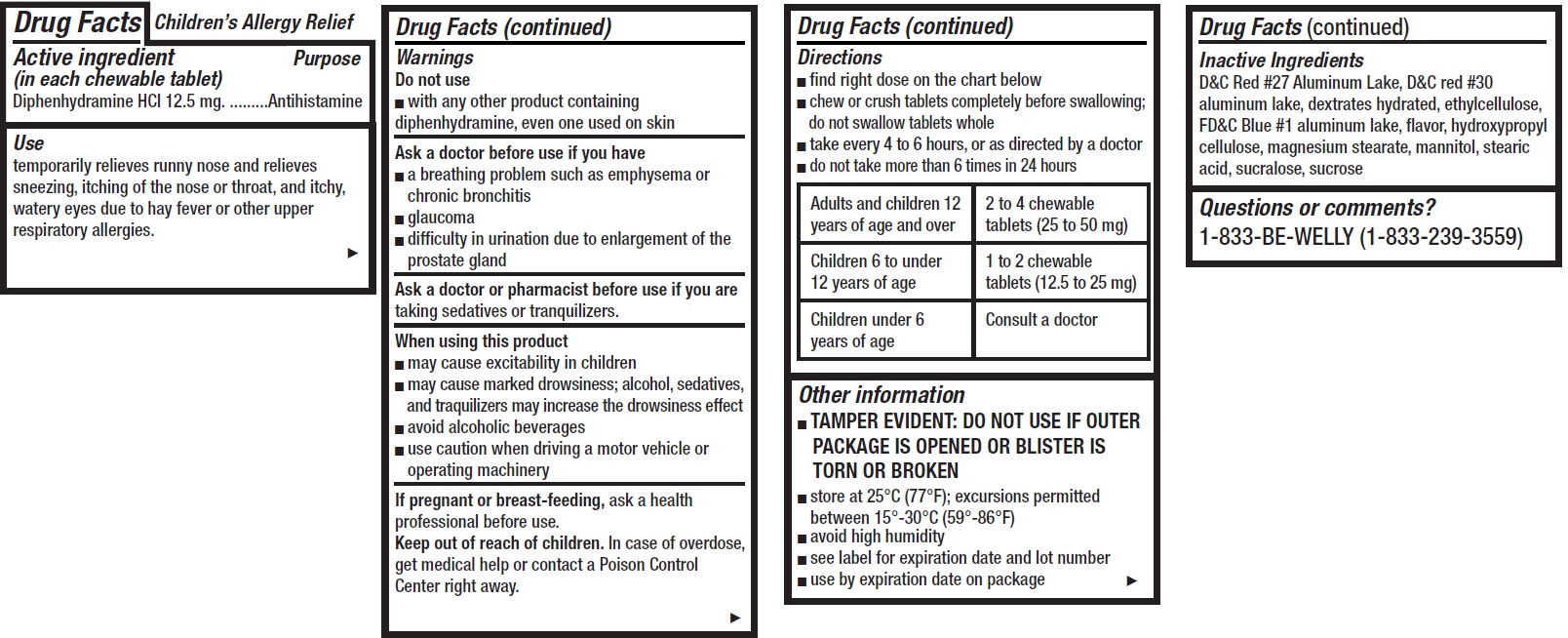
- Children's Allergy Relief, 12 tablets
- Drug Facts
- Active ingredient (in each chewable tablet)
- Use
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
-
Directions
- find right dose on the chart below
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
Adults and children 12 years of age and over 2 to 4 chewable tablets (25 to 50 mg) Children 6 to under 12 years of age 1 to 2 chewable tablets (12.5 to 25 mg) Children under 6 years of age Consult a doctor - Other information
- Inactive Ingredients
- Questions or comments?
- Package Labeling:(72663-203-49)
- Package Labeling:(72663-233-49)
- Package Labeling:(72663-225-49)
- Package Labeling:(72663-347-49)
-
INGREDIENTS AND APPEARANCE
WELLY CHILDRENS TRAVEL MEDICINE
acetaminophen, calcium carbonate, diphenhydramine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72663-203 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-203-49 1 in 1 KIT 04/06/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 BLISTER PACK 12 Part 2 2 BLISTER PACK 12 Part 3 2 BLISTER PACK 12 Part 1 of 3 CHILDRENS PAIN AND FEVER RELIEF
acetaminophen tablet, chewableProduct Information Item Code (Source) NDC:72663-233 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE (UNII: 2S7830E561) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C RED NO. 30 (UNII: 2S42T2808B) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) METHYL SALICYLATE (UNII: LAV5U5022Y) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color purple Score 2 pieces Shape ROUND Size 16mm Flavor Imprint Code 44449 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-233-49 2 in 1 KIT 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/06/2020 Part 2 of 3 CHILDRENS STOMACH SOOTHER
calcium carbonate tablet, chewableProduct Information Item Code (Source) NDC:72663-225 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 400 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYL SALICYLATE (UNII: LAV5U5022Y) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color pink Score no score Shape ROUND Size 16mm Flavor Imprint Code 44596 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-225-49 2 in 1 KIT 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 04/06/2020 Part 3 of 3 CHILDRENS ALLERGY RELIEF
diphenhydramine hydrochloride tablet, chewableProduct Information Item Code (Source) NDC:72663-347 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) METHYL SALICYLATE (UNII: LAV5U5022Y) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) Product Characteristics Color purple Score no score Shape ROUND Size 12mm Flavor Imprint Code 44585 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72663-347-49 2 in 1 KIT 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/06/2020 Labeler - Welly Health PBC (116766884)