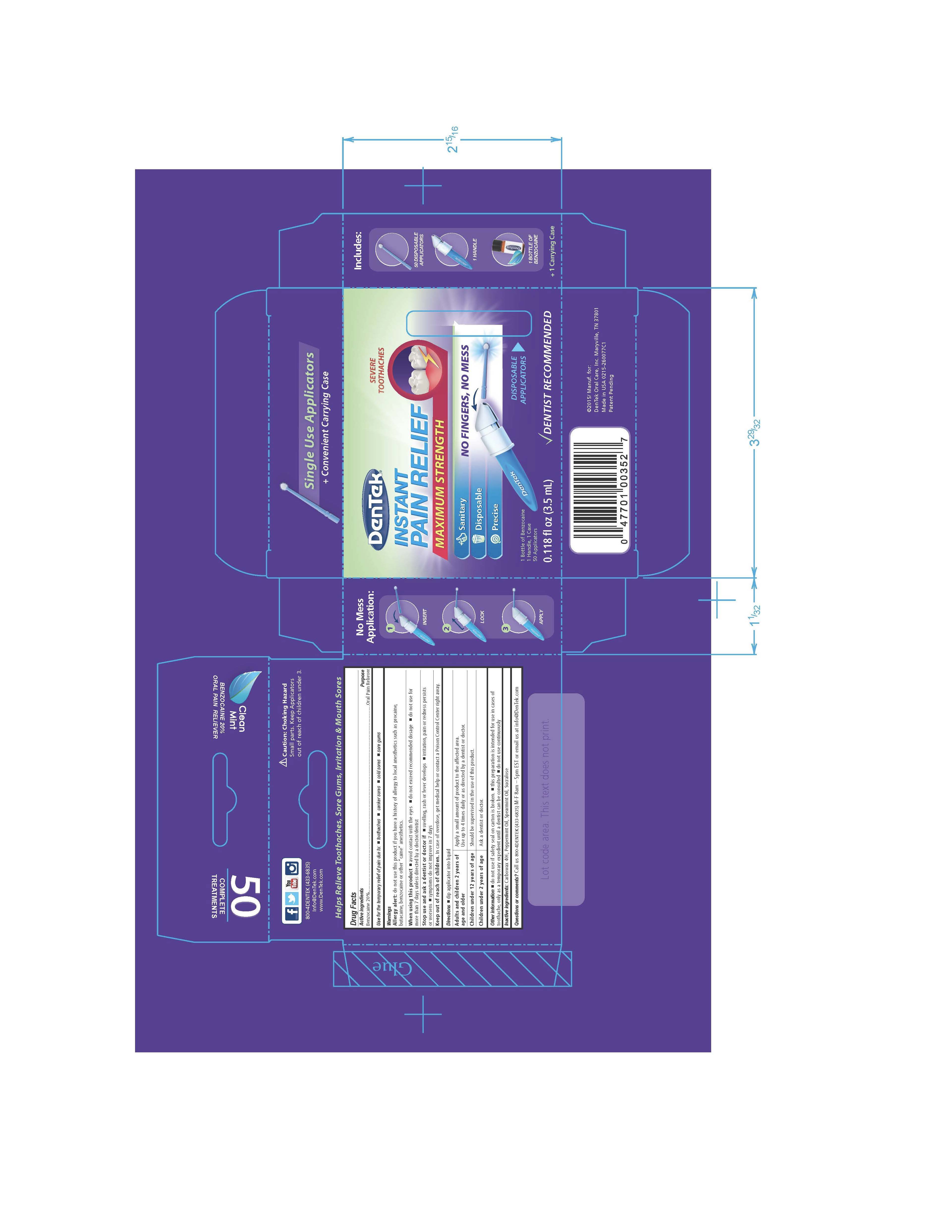
Label: DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH- benzocaine liquid
- NDC Code(s): 83062-410-03, 83062-410-04
- Packager: IIMED MEDICAL MEXICANA S. DE R.L. DE C.V
- This is a repackaged label.
- Source NDC Code(s): 60630-077
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Allergy Alert: do not use this product if you have a history of allery to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product
- avoid contact with the eyes
- do not exceed recommended dosage
- do not use for more than 7 days unless than directed by a doctor/dentist
-
Directions
dip applicator into liquid
Adults and children 2 years of age and older: Apply a small amount of product to the affected area. Use up to 4 times daily or as directed by a dentist or doctor.
Children under 12 years of age: Should be supervised in the use of this product.
Children under 2 years of age: Ask a dentist or doctor.
- Other information
- Inactive ingredients
- Questions or comments?
- DenTek Instant Pain Relief Inner Vial Label
-
INGREDIENTS AND APPEARANCE
DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83062-410(NDC:60630-077) Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength PEPPERMINT OIL (UNII: AV092KU4JH) 2.2 mg in 1 mL SUCRALOSE (UNII: 96K6UQ3ZD4) 10 mg in 1 mL SPEARMINT OIL (UNII: C3M81465G5) 3.3 mg in 1 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 784.5 mg in 1 mL Product Characteristics Color yellow (Colorless to light yellow liquid) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83062-410-04 1 in 1 CARTON 10/10/2022 1 NDC:83062-410-03 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 10/10/2022 Labeler - IIMED MEDICAL MEXICANA S. DE R.L. DE C.V (812894376) Establishment Name Address ID/FEI Business Operations IIMED MEDICAL MEXICANA S. DE R.L. DE C.V 812894376 relabel(83062-410) , repack(83062-410)