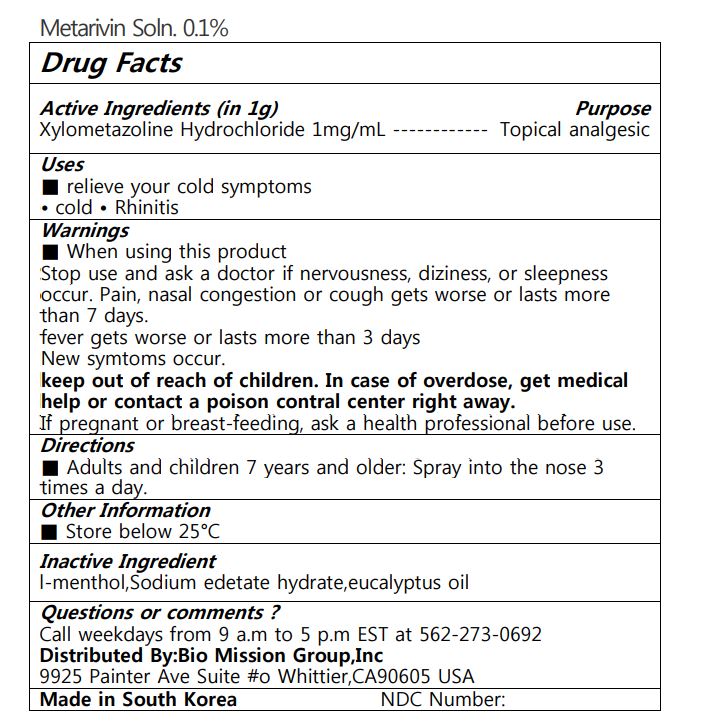
Label: METARIVIN SOLN. 0.1%- xylometazoline hydrochloride liquid
- NDC Code(s): 72988-0030-1
- Packager: Lydia Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
■ When using this product Stop use and ask a doctor if nervousness, diziness, or sleepness occur. Pain, nasal congestion or cough gets worse or lasts more than 7 days. fever gets worse or lasts more than 3 days New symtoms occur. keep out of reach of children. In case of overdose, get medical help or contact a poison contral center right away. If pregnant or breast-feeding, ask a health professional before use.
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METARIVIN SOLN. 0.1%
xylometazoline hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72988-0030 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLOMETAZOLINE HYDROCHLORIDE (UNII: X5S84033NZ) (XYLOMETAZOLINE - UNII:WPY40FTH8K) XYLOMETAZOLINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength COBALT DISODIUM EDETATE (UNII: 3EY1Y2QRLI) EUCALYPTUS OIL (UNII: 2R04ONI662) LEVOMENTHOL (UNII: BZ1R15MTK7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72988-0030-1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/30/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/30/2022 Labeler - Lydia Co., Ltd. (695735569) Registrant - Lydia Co., Ltd. (695735569) Establishment Name Address ID/FEI Business Operations Lydia Co., Ltd. 695735569 manufacture(72988-0030)