Label: TOTY SOLARIA MINERAL- titanium dioxide, zinc oxide cream
- NDC Code(s): 82141-3161-1, 82141-3161-2, 82141-3161-3, 82141-3161-4
- Packager: Chipican LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Shake well before use
- Apply liberally 15 minutes before sun exposure
- Reapply:
After 80 minutes of swimming or sweating
Immediately after towel drying
At least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive Ingredients
Water, Dimethicone, Isododecane, C12-15 Alkyl Benzoate, Hydrogenated Didecene, Butyloctyl Salicylate, Aluminum Hydroxide, Styrene/Acrylates Copolymer, Phenethyl Benzoate, PEG-9 Polydimethylsiloxyethyl Dimethicone, Nylon-12, Calaguala ( Polypodium leucotomos) Leaf Extract, Ferulic Acid, 3-O-Ethyl Ascorbic Acid, Tocopheryl Acetate, Tocopherol, Cutleaf Grouncherry (Physalis angulata) Extract, Green Tea ( Camellia sinensis) Extract, Plankton ( Chlamydomonas reinhardti) Extract, Arginine, Melanin, Caprylyl Glycol, Isohexadecane, Disteardimonium Hectorite, Hydrogen Dimethicone, Phenylpropanol, Polyhydroxystearic Acid, Magnesium Sulfate, Dimethicone/PEG-10/15 Crosspolymer, Propanediol, Triethoxycaprylylsilane, Propylene Carbonate, Caprylic/Capric Triglyceride, Panthenyl Triacetate, Butylene Glycol, Propylene Glycol, Ethyl Linoleate, Oleyl Alcohol, Pentylene Glycol, Oxothiazolidine, Dipropylene Glycol, Sodium Citrate, Palmitoyl Hydroxypropyltrimonium Amylopectin/Glycerin Crosspolymer, 1,2-Hexanediol, Lecithin, Sodium Benzoate, Hydrogenated Lecithin, Phenoxyethanol, Ethylhexylglycerin, Iron Oxides (Ferric Oxide Red (CI 77491), Ferric Oxide Yellow (CI 77491), Ferrosoferric Oxide (CI 77499)).
-
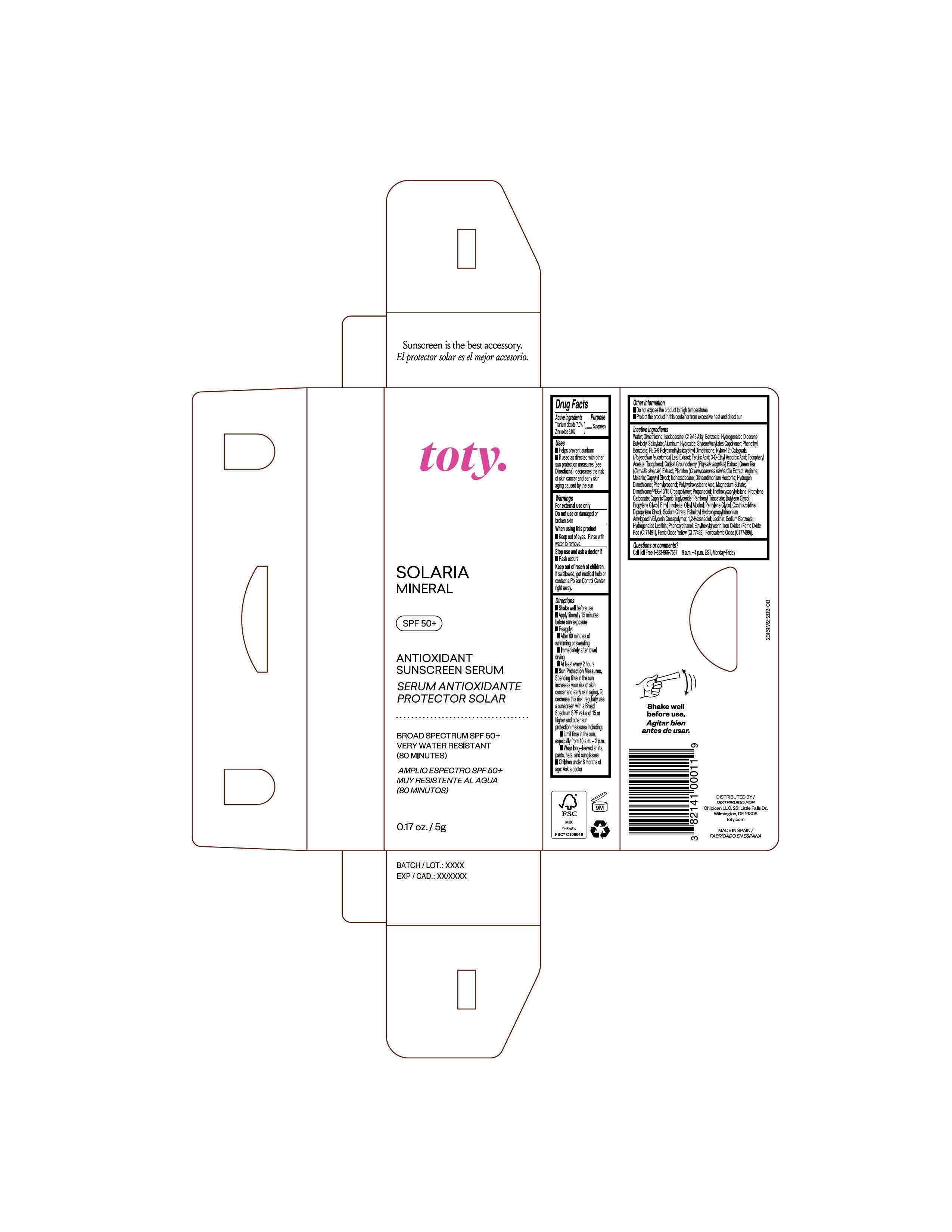
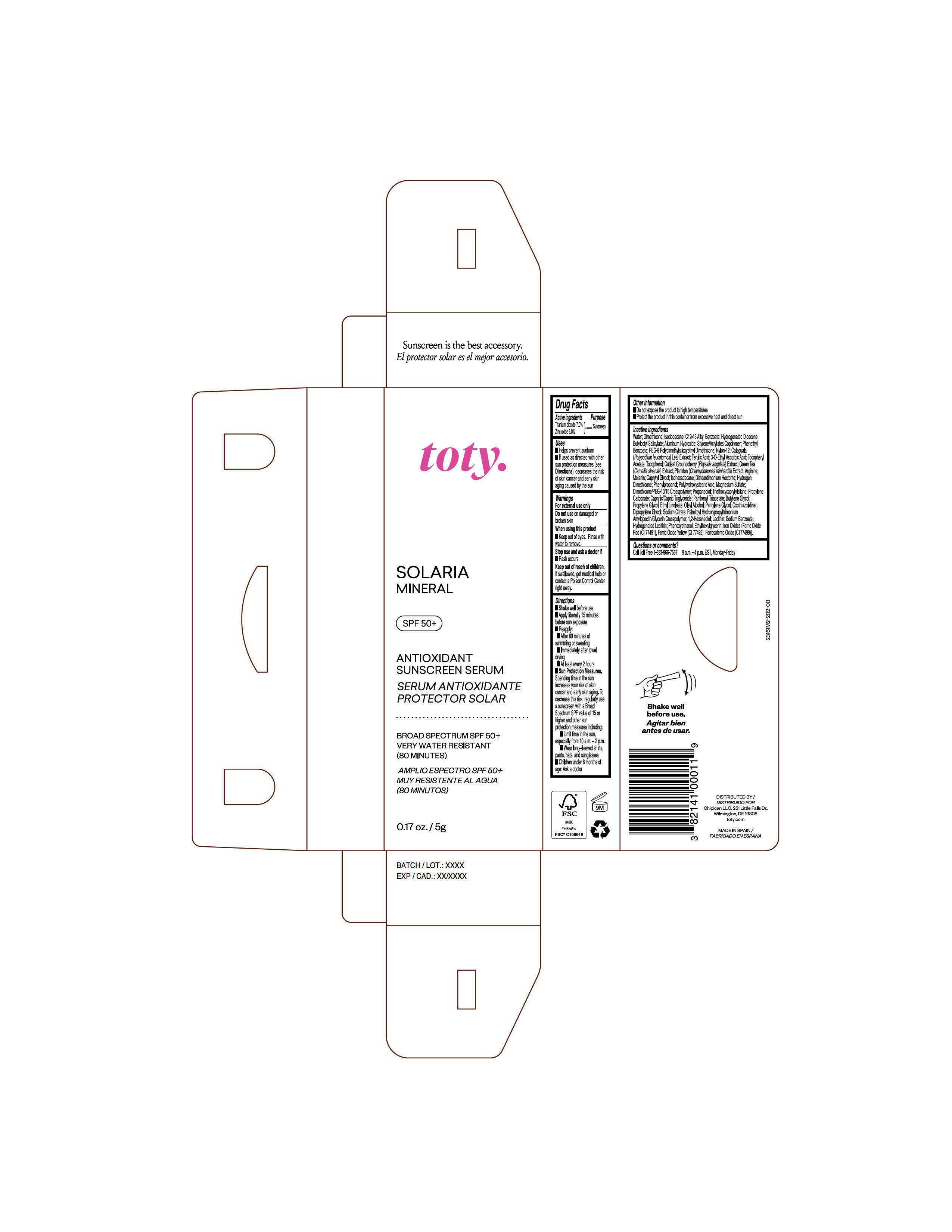
Package Label.Principal Display Panel-
Sample
NDC 82141-3161-2 (toty Solaria Mineral, Sample)
toty.
SOLARIA
MINERAL
SPF 50+
ANTIOXIDANT
SUNSCREEN SERUM
SERUM ANTIOXIDANTE
PROTECTOR SOLAR
.....................................................................................
BROAD SPECTRUM SPF 50+
VERY WATER RESISTANT
(80 MINUTES)
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA
(80 MINUTOS)
0.17 oz/5g
-
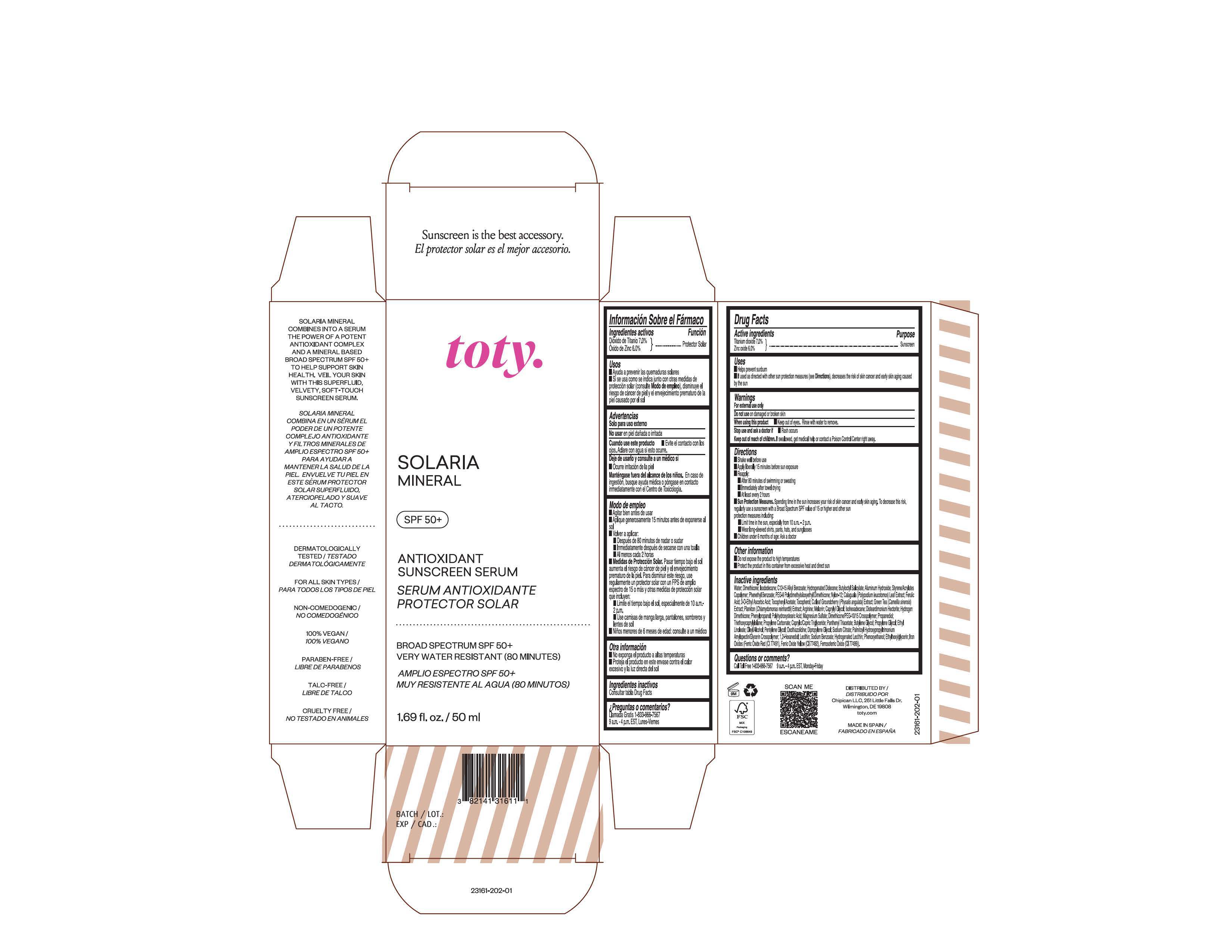
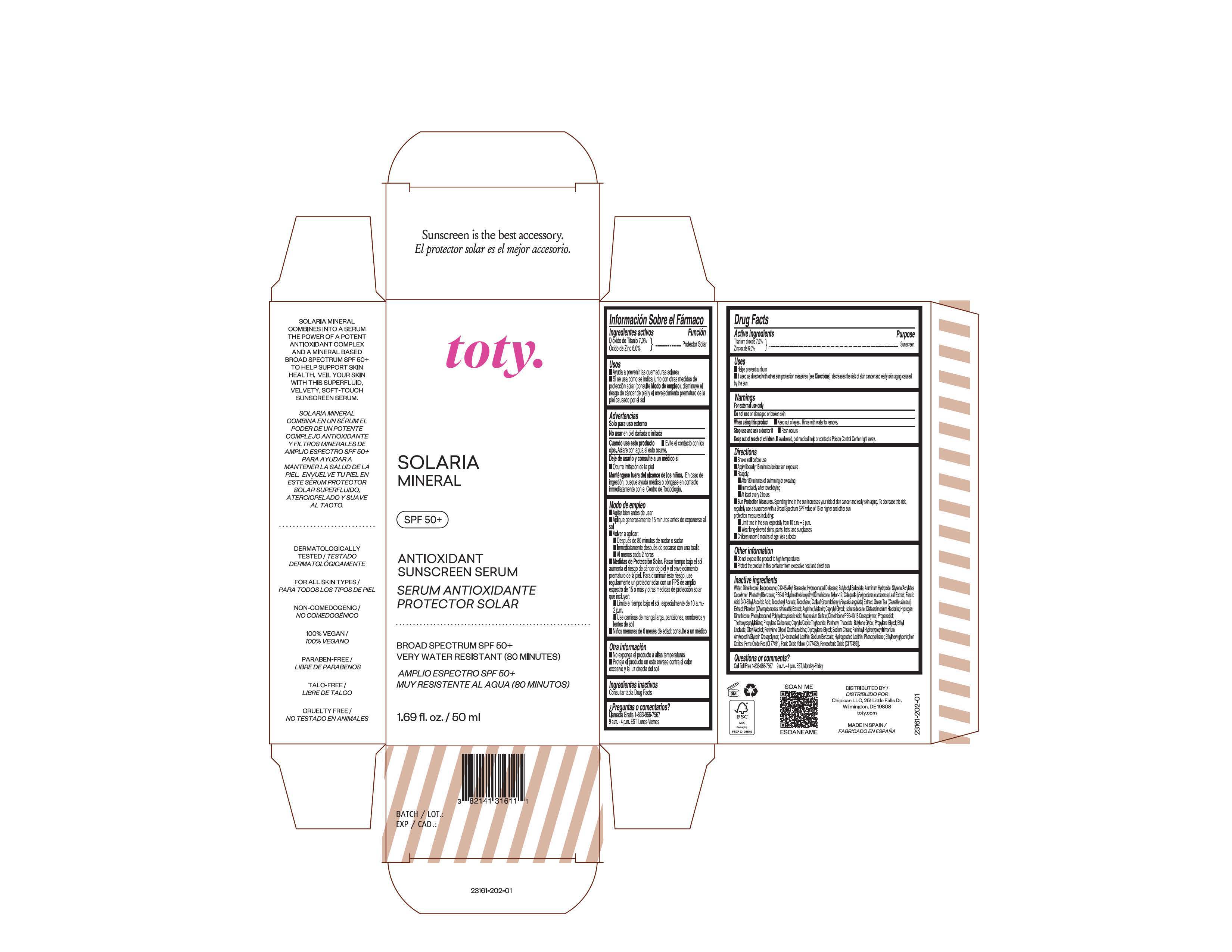
Package Label.Principal Display Panel
NDC 82141-3161-1 (toty Solaria Mineral)
toty.
SOLARIA
MINERAL
SPF 50+
ANTIOXIDANT
SUNSCREEN SERUM
SERUM ANTIOXIDANTE
PROTECTOR SOLAR
.....................................................................................
BROAD SPECTRUM SPF 50+
VERY WATER RESISTANT (80 MINUTES)
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
1.69 fl. oz/50 mL
-
Package Label. Princip[al Display Panel-
Travel Size)
NDC 82141-3161-3 (toty Solaria Mineral, Travel Size)
toty.
SOLARIA
MINERAL
SPF 50+
ANTIOXIDANT
SUNSCREEN SERUM
SERUM ANTIOXIDANTE
PROTECTOR SOLAR
.....................................................................................
BROAD SPECTRUM SPF 50+
VERY WATER RESISTANT (80 MINUTES)
AMPLIO ESPECTRO SPF 50+
MUY RESISTENTE AL AGUA (80 MINUTOS)
1.0 fl. oz/30 mL

-
INGREDIENTS AND APPEARANCE
TOTY SOLARIA MINERAL
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-3161 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 7 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6 g in 100 g Inactive Ingredients Ingredient Name Strength PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ISOHEXADECANE (UNII: 918X1OUF1E) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OXOTHIAZOLIDINE (UNII: M6U1ZG59XD) FERRIC OXIDE RED (UNII: 1K09F3G675) NYLON-12 (UNII: 446U8J075B) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 3-PHENYL-1-PROPANOL (UNII: U04IC2765C) HYDROGENATED DIDECENE (UNII: 048B98MT5O) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENETHYL BENZOATE (UNII: 0C143929GK) TOCOPHEROL (UNII: R0ZB2556P8) PHYSALIS ANGULATA (UNII: W4TKW9D5GG) PROPANEDIOL (UNII: 5965N8W85T) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ETHYL LINOLEATE (UNII: MJ2YTT4J8M) OLEYL ALCOHOL (UNII: 172F2WN8DV) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) WATER (UNII: 059QF0KO0R) ISODODECANE (UNII: A8289P68Y2) ARGININE (UNII: 94ZLA3W45F) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) FERULIC ACID (UNII: AVM951ZWST) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) DIPROPYLENE GLYCOL (UNII: E107L85C40) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) SODIUM BENZOATE (UNII: OJ245FE5EU) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) ANHYDROUS TRISODIUM CITRATE (UNII: RS7A450LGA) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) CHLAMYDOMONAS REINHARDTII (UNII: QS58JP94CS) MELANIN SYNTHETIC (TYROSINE, PEROXIDE) (UNII: O0CV1RMR44) DIMETHICONE (UNII: 92RU3N3Y1O) PALMITOYL HYDROXYPROPYLTRIMONIUM AMYLOPECTIN/GLYCERIN CROSSPOLYMER (UNII: 7F9WLW26M2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-3161-1 1 in 1 CARTON 06/01/2023 01/31/2024 1 50 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:82141-3161-2 1 in 1 CARTON 06/01/2023 2 5 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:82141-3161-3 1 in 1 CARTON 01/08/2024 3 30 g in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:82141-3161-4 1 in 1 CARTON 06/01/2023 4 50 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - Chipican LLC (118132015) Establishment Name Address ID/FEI Business Operations Industrial Farmaceutica Cantabria SA 470471158 manufacture(82141-3161) , label(82141-3161) , pack(82141-3161)