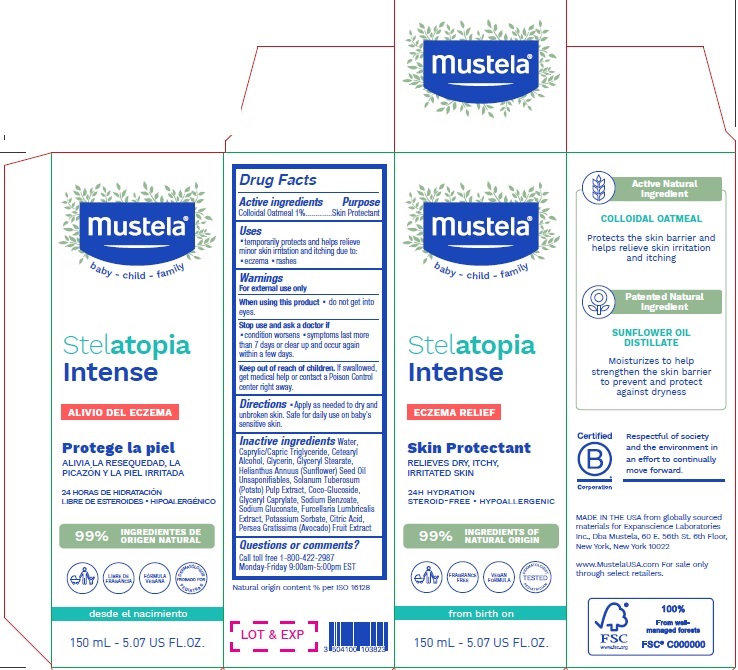
Label: MUSTELA STELATOPIA INTENSE ECZEMA RELIEF SKIN PROTECTANT- colloidal oatmeal lotion
- NDC Code(s): 64768-7024-1, 64768-7024-2
- Packager: Expanscience Laboratories d/b/a Mustela
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water, Caprylic/Capric Triglyceride, Cetearyl Alcohol, Glycerin, Glyceryl Stearate, Helianthus Annuus (Sunfower) Seed Oil Unsaponifables, Solanum Tuberosum (Potato) Pulp Extract, Coco-Glucoside, Glyceryl Caprylate, Sodium Benzoate, Sodium Gluconate, Furcellaria Lumbricalis Extract, Potassium Sorbate, Citric Acid, Persea Gratissima (Avocado) Fruit Extract
- Questions or comments?
- Company Information
- Product Packaging
-
INGREDIENTS AND APPEARANCE
MUSTELA STELATOPIA INTENSE ECZEMA RELIEF SKIN PROTECTANT
colloidal oatmeal lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64768-7024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength POTATO PULP (UNII: 844I2DX0IS) SODIUM BENZOATE (UNII: OJ245FE5EU) FURCELLERAN (UNII: 30QS0PF14U) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) AVOCADO (UNII: SDS87L369F) GLYCERIN (UNII: PDC6A3C0OX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCO GLUCOSIDE (UNII: ICS790225B) SODIUM GLUCONATE (UNII: R6Q3791S76) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64768-7024-1 30 mL in 1 TUBE; Type 0: Not a Combination Product 10/01/2022 2 NDC:64768-7024-2 1 in 1 CARTON 10/01/2022 2 150 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 Labeler - Expanscience Laboratories d/b/a Mustela (181191057)