Label: TINEACIDE ANTIFUNGAL- miconazole nitrate cream
- NDC Code(s): 63347-502-01
- Packager: Blaine Labs Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Keep out of reach of children
- Uses
- Warnings
- When using this product,
- Stop use and consult a physician if
-
Directions
- Adult and children 2 years and older. Children under 2 years: ask physician.
- Wash the affected area with soap and water and dry thoroughly before applying.
- For athlete's foot between the toes: apply to affected skin between and around the toes twice a day for 4 weeks (morning and night), or as directed by a physician.Wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- For jock itch and ringworm apply twice daily to affected skin for 2 weeks or as directed by a physician. This product is not effective on the scalp or mails.
- Wash hands after each use.
- Other Information
- Inactive Ingredients
-
Product Label
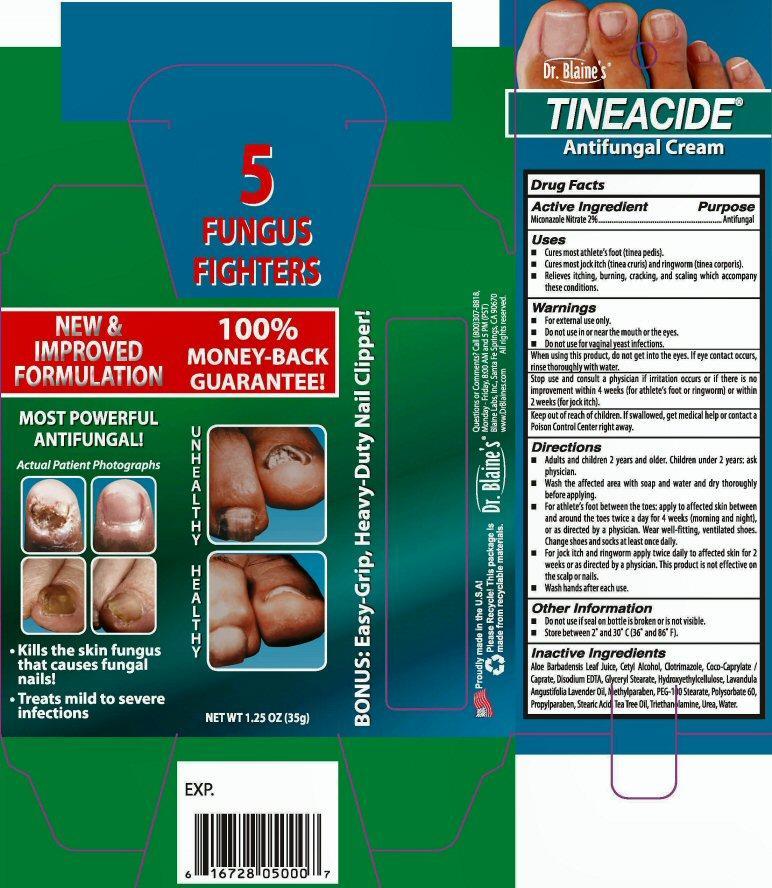
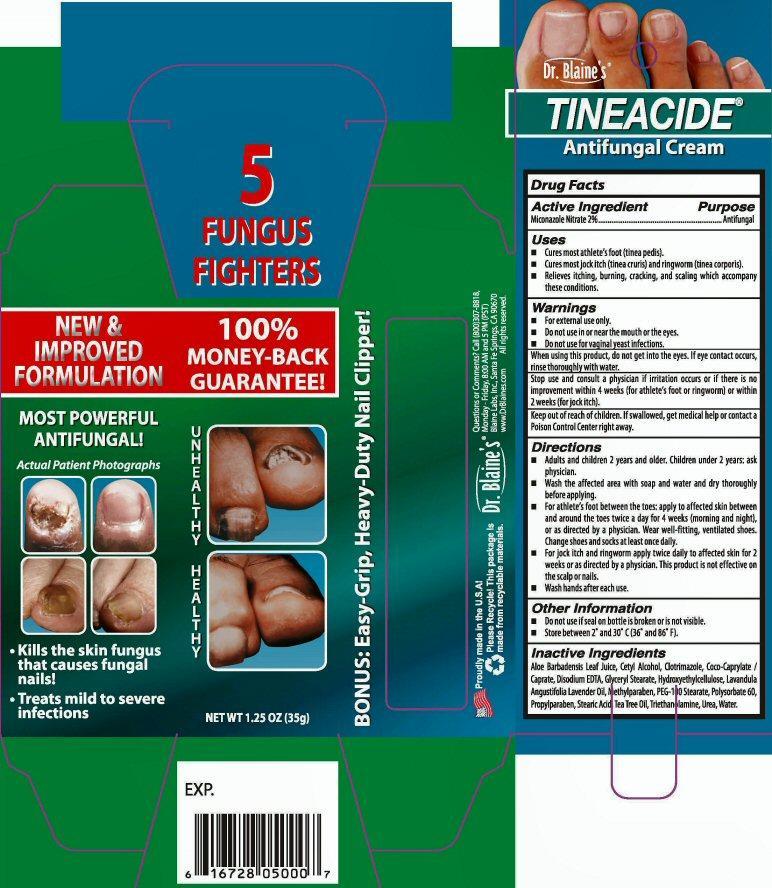
Dr. Blaine's
TINEACIDE®
Antifungal Cream
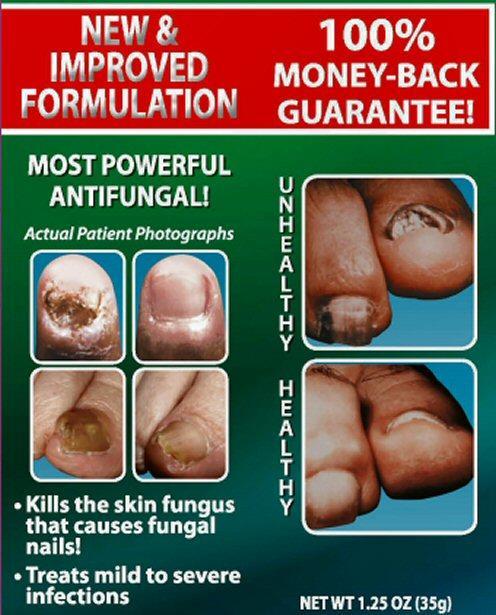
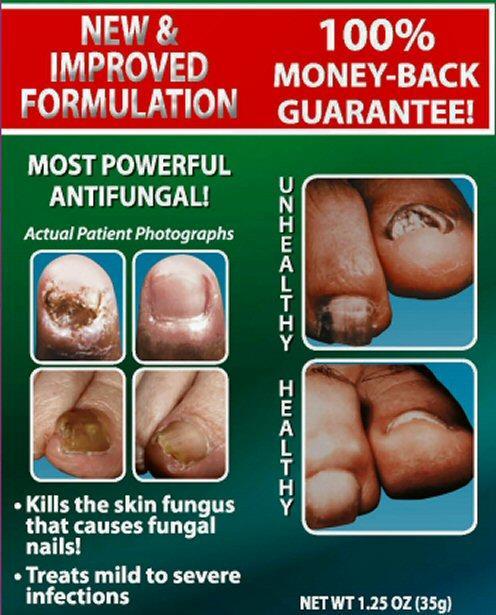
Kills the skin fungus that causes fungal nails
Treats mild to severe infections
NET WT 1.25 OZ (35 g)
5 FUNGUS FIGHTERS
NEW and IMPROVED FORMULATION 100% MONEY-BACK GUARANTEE!
BONUS: Easy-Grip, Heavy-Duty Nail Clipper!
EXP.
Proudly made in the U.S.A.!
Please Recycle! This package is made from recyclable materials.
Questions or Comments? Call (800) 307-8818 Monday-Friday 8:00AM and 5:00PM (EST)
Blaine Labs Inc. Santa Fe Springs, CA 90670 www.DrBlaines.com All rights reserved.
res
-
INGREDIENTS AND APPEARANCE
TINEACIDE ANTIFUNGAL
miconazole nitrate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63347-502 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 0.7 g in 35 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) CLOTRIMAZOLE (UNII: G07GZ97H65) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYETHYL CELLULOSE (140 MPA.S AT 5%) (UNII: 8136Y38GY5) LAVENDER OIL (UNII: ZBP1YXW0H8) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-100 STEARATE (UNII: YD01N1999R) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARIC ACID (UNII: 4ELV7Z65AP) TEA TREE OIL (UNII: VIF565UC2G) TROLAMINE (UNII: 9O3K93S3TK) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63347-502-01 35 g in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/01/2013 Labeler - Blaine Labs Inc. (017314571) Registrant - Blaine Labs Inc. (017314571) Establishment Name Address ID/FEI Business Operations Blaine Labs Inc. 017314571 manufacture(63347-502)