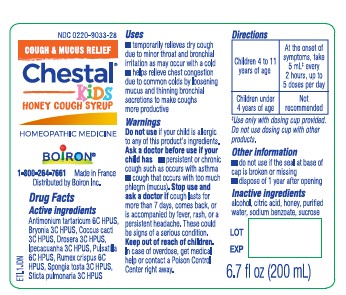
Label: CHESTAL FOR CHILDREN- antimony potassium tartrate, bryonia alba root, protortonia cacti, drosera rotundifolia, ipecac, pulsatilla vulgaris, rumex crispus root, pongia officinalis skeleton, roasted, lobaria pulmonaria syrup
- NDC Code(s): 0220-9033-15, 0220-9033-16, 0220-9033-28
- Packager: Laboratoires Boiron
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 18, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Antimonium tartaricum 6C HPUS (12.35 mg) (contains less than 10 -11 mg antimony)
Bryonia 3C HPUS (12.35 mg)
Coccus cacti 3C HPUS (12.35 mg)
Drosera 3C HPUS (12.35 mg)
Ipecacuanha 3C HPUS (12.35 mg) (contains less than 10 -6 mg emetine alkaloids)
Pulsatilla 6C HPUS (12.35 mg)
Rumex crispus 6C HPUS (12.35 mg) (contains less than 10 -13 mg anthraquinone glycosides)
Spongia tosta 3C HPUS (12.35 mg)
Sticta pulmonaria 3C HPUS (12.35 mg)The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Parmacopoeia of the United States.
-
PURPOSE
Antimonium tartaricum 6C HPUS .. Helps loosen thick mucus
Bryonia 3C HPUS ... Relieves dry and painful cough
Coccus cacti 3C HPUS ... Relieves cough associated with a tickling in the throat
Drosera 3C HPUS ... Relieves barking cough worse at night
Ipecacuanha 3C HPUS ... Relieves cough associated with nausea
Pulsatilla 6C HPUS ... Relieves wet cough during the day becoming dry at night
Rumex crispus 6C HPUS ... Relieves dry cough triggered by cold air
Spongia tosta 3C HPUS ... Relieves dry, croupy and barking cough
Sticta pulmonaria 3C HPUS ... Relieves nighttime hacking cough - INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
do not use if glued carton end flaps are open or if the seal at base of cap is broken or missing
retain this carton for full directions
contains 4 g of sugar per 5 ml
contains 0.00007% alcohol
dispose 1 year after opening
6.7 fl oz (200 ml)
Cough & Mucus Relief*
Chest Congestion, Dry, Fitful Cough*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
- INACTIVE INGREDIENT
- QUESTIONS
- PREGNANCY OR BREAST FEEDING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHESTAL FOR CHILDREN
antimony potassium tartrate, bryonia alba root, protortonia cacti, drosera rotundifolia, ipecac, pulsatilla vulgaris, rumex crispus root, pongia officinalis skeleton, roasted, lobaria pulmonaria syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0220-9033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 3 [hp_C] PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 3 [hp_C] DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 3 [hp_C] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 3 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_C] RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 6 [hp_C] SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 3 [hp_C] LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 3 [hp_C] ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_C] Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) HONEY (UNII: Y9H1V576FH) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor HONEY (Honey) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0220-9033-28 1 in 1 BOX; Type 0: Not a Combination Product 08/01/1996 2 NDC:0220-9033-16 1 in 1 BOX; Type 0: Not a Combination Product 08/01/1996 01/31/2017 3 NDC:0220-9033-15 1 in 1 BOX; Type 0: Not a Combination Product 08/01/1996 01/31/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/1996 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9033)