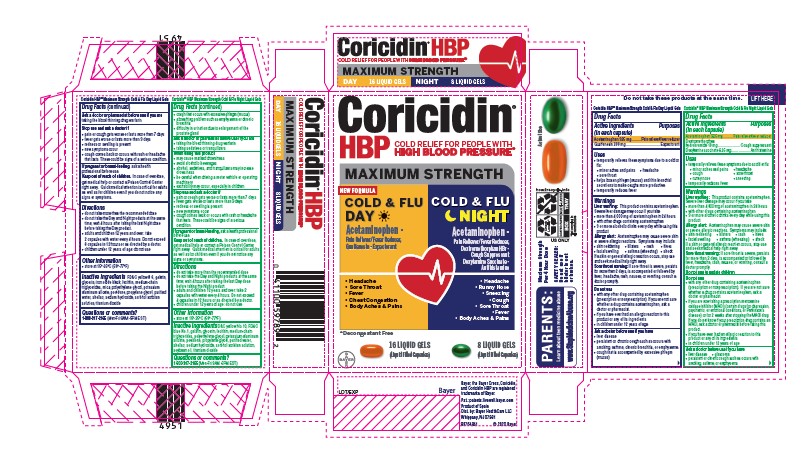
Label: CORICIDIN HBP MAXIMUM STRENGTH COLD AND FLU DAY AND NIGHT- acetaminophen, dextromethorphan hydrobromide, guaifenesin, doxylamine succinate kit
- NDC Code(s): 11523-0100-1
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
Do not use
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
Aska a doctor
Ask a doctor before use if you have
● liver disease
● persistent or chronic cough such as occurs with smoking, asthma,chronic bronchitis,
or emphysema
● cough that occurs with excess phelgm
Ask a doctor or pharmacist
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
-
Directions
Directions
· do not take more than the recommended dose
· do not take the Day and Night products at the same time; wait 4
hours after taking the last Night dose before taking the Day product.
· adults and children 12 years and over: take 2 capsules with water
every 4 hours. Do not exceed 6 capsules in 12 hours or as
directed by a doctor.
· children under 12 years of age: do not use
- Other information
- Inactive ingrediens
- Questions or comments?
- Coricidin HBP Maximum Stregth Cold & Flu Night Liquid Gels
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
Liver Warning
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
-
DO NOT USE
Do not use to sedate children
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
- ASK DOCTOR
-
ASK DOCTOR/PHARMACIST
Ask a doctor before use if you have
● liver disease ● glaucoma
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
● cough that occurs with excessive phlegm (mucus)
● a breathing problem such as emphysema or chronic bronchitis
● difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
● taking the blood thinning drug warfarin
● taking sedatives or tranquilizers - WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
· do not take more than the recommended dose
· do not take the Day and Night products at the same time; wait 4
hours after taking the last Day dose before taking the Night product.
· adults and children 12 years and over: take 2 capsules with water
every 4 hours. Do not exceed 4 capsules in 12 hours or as
directed by a doctor.
· children under 12 years of age: do not use
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
 Coricidin® HBP
Coricidin® HBP
COLD RELIEF FOR PEOPLE WITH HIGH BLOOD PRESSUREƗ
MAXIMUM STRENGTH
COLD & FLU DAY
ACETAMINOPHEN - Pain Reliever/Fever Reducer
GUAIFENESIN - Expectorant
Relieves
- Body Aches & Pains
- Headache
- Chest Congestion
- Sore Throat
- Fever
Ɨ Decongestant Free
16 LIQUID GELS
(Liquid Filled Capsules)
COLD & FLU NIGHT
DOXYLAMINE SUCCINATE - Antihistamine
ACETAMINOPHEN - Pain Reliever/Fever Reducer
DEXTROMETHORPHAN HBr- Cough Suppressant
RELIEVES
- Body Aches & Pains
- Headache
- Runny Nose & Sneezing
- Cough
- Sore Throat
- Fever
Ɨ Decongestant Free
8 LIQUID GELS
(Liquid Filled Capsules)
-
INGREDIENTS AND APPEARANCE
CORICIDIN HBP MAXIMUM STRENGTH COLD AND FLU DAY AND NIGHT
acetaminophen, dextromethorphan hydrobromide, guaifenesin, doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-0100 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-0100-1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 BLISTER PACK 8 Part 2 2 BLISTER PACK 4 Part 1 of 2 CORICIDIN HBP MAXIMUM STRENGTH COLD AND FLU DAY
acetaminophen, guaifenesin capsule, liquid filledProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SHELLAC (UNII: 46N107B71O) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN (UNII: 6O92ICV9RU) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM ALUMINUM DISILICATE (UNII: SRB14JRX6C) POVIDONE (UNII: FZ989GH94E) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color orange Score no score Shape OVAL (oblong) Size 18mm Flavor Imprint Code CHBPD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/01/2020 Part 2 of 2 CORICIDIN HBP MAXIMUM STRENGTH COLD AND FLU NIGHT
acetaminophen, doxylamine succinate, dextromethorphan hydrobromide, doxylamine succinate capsule, liquid filledProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SHELLAC (UNII: 46N107B71O) SORBITAN (UNII: 6O92ICV9RU) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POVIDONE (UNII: FZ989GH94E) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITOL (UNII: 506T60A25R) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color green Score no score Shape OVAL Size 17mm Flavor Imprint Code CHBPN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/01/2020 Labeler - Bayer HealthCare LLC. (112117283)