Label: EQUATE THERAPEUTIC DANDRUFF EXTRA STRENGTH ANTI-DANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS- coal tar shampoo
- NDC Code(s): 79903-088-01
- Packager: Walmart Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
When using this product
- avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water
- use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application.
- do not use for prolonged periods without consulting a doctor.
- do not use this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor.
- Directions
- Other information
-
Inactive ingredients
water, sodium laureth sulfate, cocamidopropyl betaine, cocamide MEA, laureth-4, polysorbate 20, bis-hydroxyethyl dihydroxypropyl stearammonium chloride, PEG-120 methyl glucose dioleate, disodium EDTA, citric acid, phenoxyethanol, fragrance, methylchloroisothiazolinone, methylisothiazolinone, red 40, blue 1, yellow 5, yellow 6.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
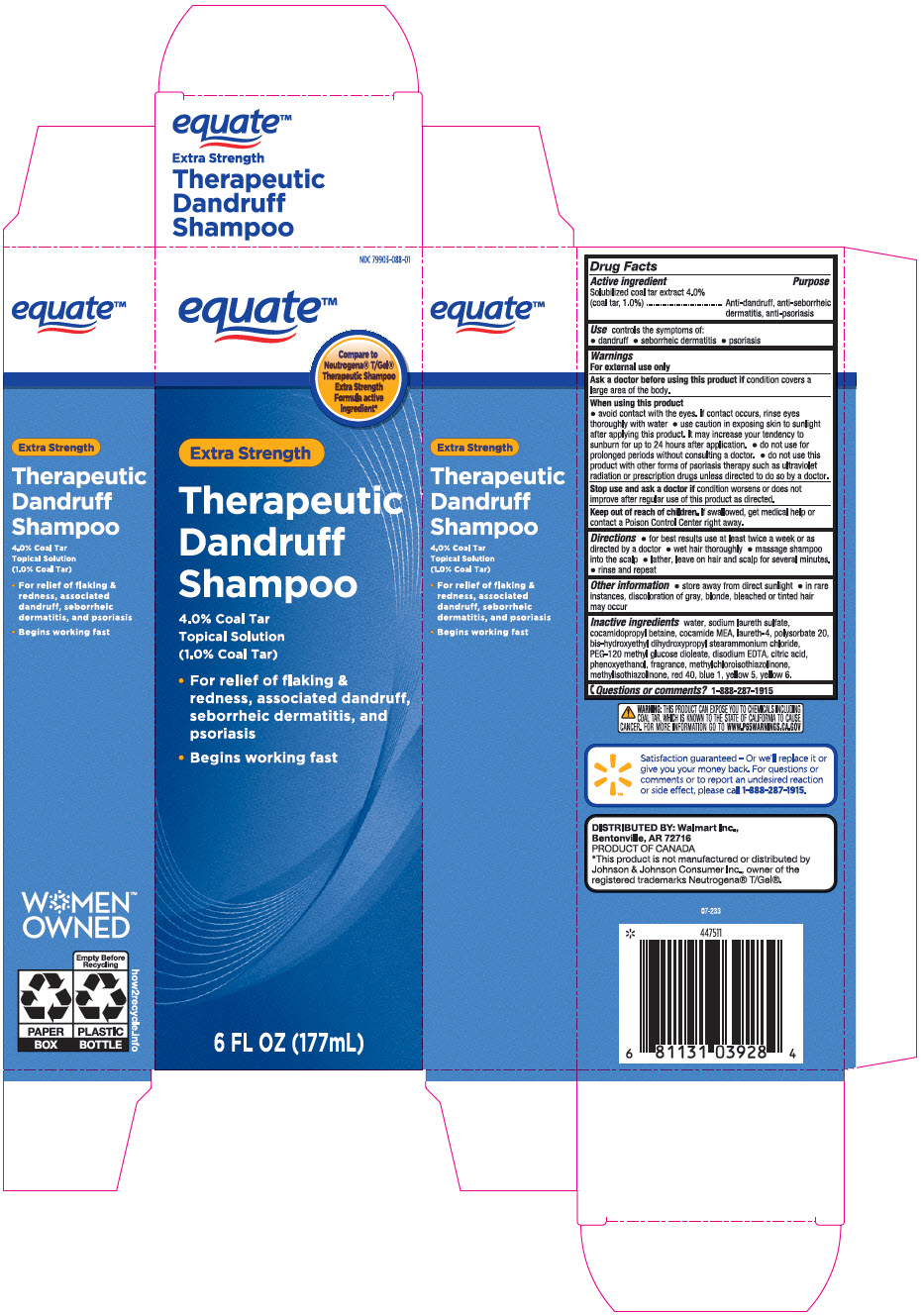
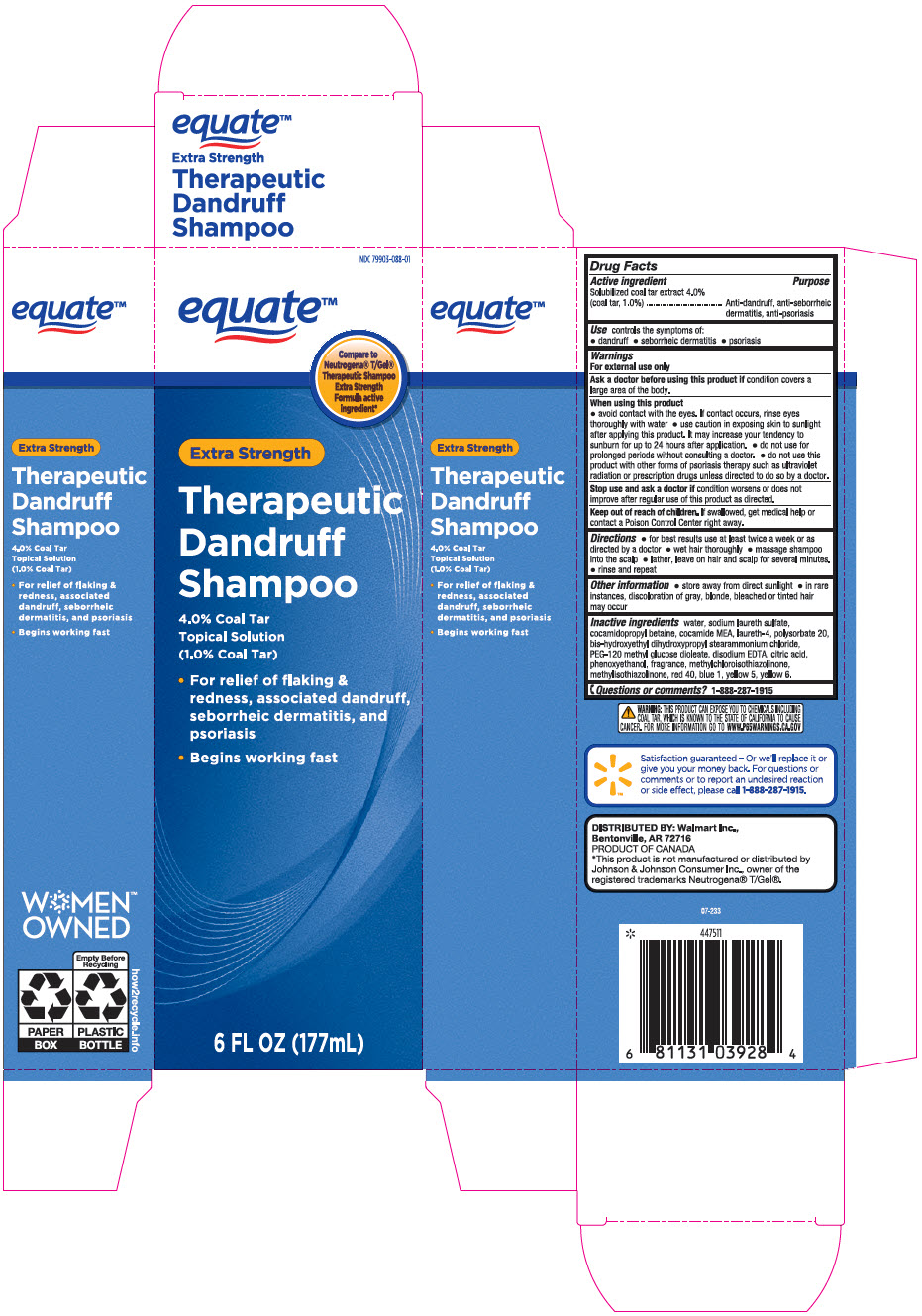
PRINCIPAL DISPLAY PANEL - 177 mL Bottle Carton
NDC 79903-088-01
equate™
Compare to
Neutrogena® T/Gel®
Therapeutic Shampoo
Extra Strength
Formula active
ingredient*Extra Strength
Therapeutic
Dandruff
Shampoo4.0% Coal Tar
Topical Solution
(1.0% Coal Tar)- For relief of flaking &
redness, associated dandruff,
seborrheic dermatitis, and
psoriasis - Begins working fast
6 FL OZ (177mL)
- For relief of flaking &
-
INGREDIENTS AND APPEARANCE
EQUATE THERAPEUTIC DANDRUFF EXTRA STRENGTH ANTI-DANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS
coal tar shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-088 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength coal tar (UNII: R533ESO2EC) (coal tar - UNII:R533ESO2EC) coal tar 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Cocamidopropyl Betaine (UNII: 5OCF3O11KX) COCO MONOETHANOLAMIDE (UNII: C80684146D) Laureth-4 (UNII: 6HQ855798J) Polysorbate 20 (UNII: 7T1F30V5YH) BIS-HYDROXYETHYL DIHYDROXYPROPYL STEARAMMONIUM CHLORIDE (UNII: 32II08O72A) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Phenoxyethanol (UNII: HIE492ZZ3T) Methylchloroisothiazolinone (UNII: DEL7T5QRPN) Methylisothiazolinone (UNII: 229D0E1QFA) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color BROWN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-088-01 1 in 1 CARTON 03/14/2022 1 177 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M032 03/14/2022 Labeler - Walmart Inc. (051957769) Registrant - Garcoa, INC (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries INC 255106239 MANUFACTURE(79903-088) , LABEL(79903-088) , PACK(79903-088)