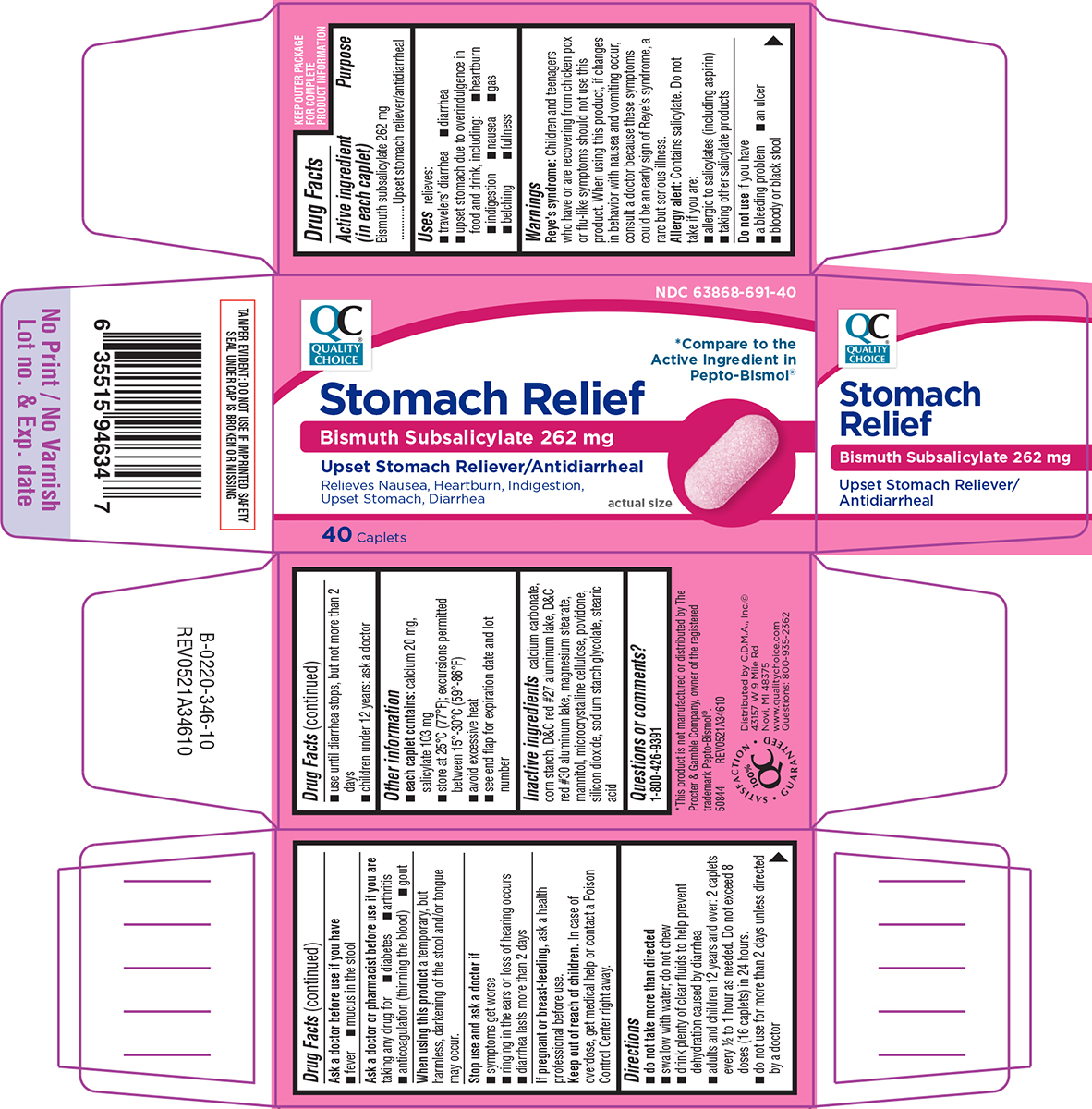
Label: STOMACH RELIEF- bismuth subsalicylate tablet
- NDC Code(s): 63868-691-40
- Packager: CHAIN DRUG MARKETING ASSOCIATION INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are:
- allergic to salicylates (including aspirin)
- taking other salicylate products
Ask a doctor or pharmacist before use if you are
taking any drug for
- diabetes
- arthritis
- anticoagulation (thinning the blood)
- gout
-
Directions
- do not take more than directed
- swallow with water; do not chew
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- adults and children 12 years and over: 2 caplets every 1/2 to 1 hour as needed. Do not exceed 8 doses (16 caplets) in 24 hours.
- do not use for more than 2 days unless directed by a doctor
- use until diarrhea stops, but not more than 2 days
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
QC®
QUALITY
CHOICENDC 63868-691-40
*Compare to the
Active Ingredient in
Pepto-Bismol®Stomach Relief
Bismuth Subsalicylate 262 mg
Upset Stomach Reliever/AntidiarrhealRelieves Nausea, Heartburn, Indigestion,
Upset Stomach, Diarrheaactual size
40 Caplets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING*This product is not manufactured or distributed by The
Procter & Gamble Company, owner of the registered
trademark Pepto-Bismol®.
50844 REV0521A34610SATISFACTION
GUARANTEED
100% QCDistributed by C.D.M.A., Inc.©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362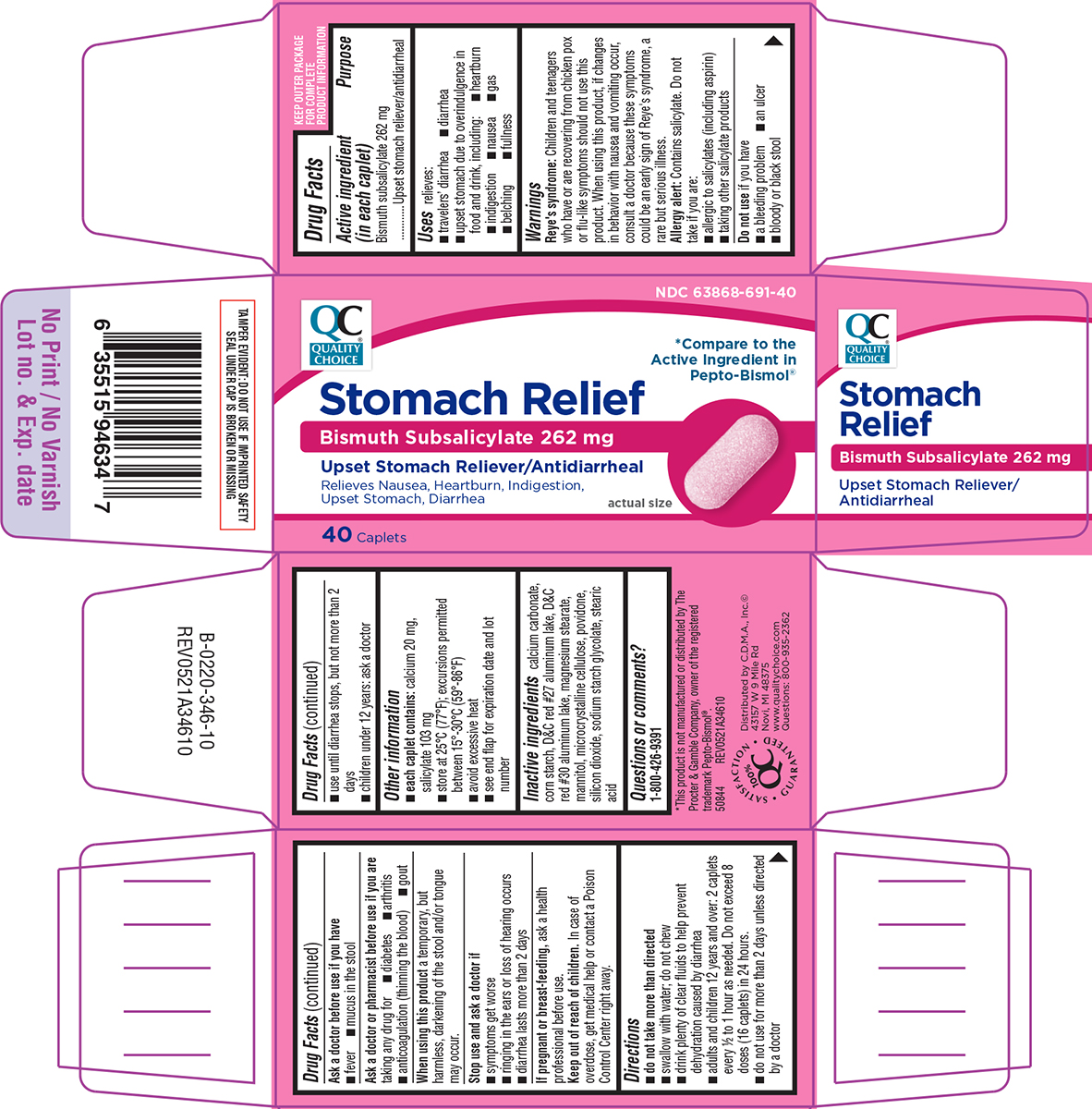
Quality Choice 44-346
-
INGREDIENTS AND APPEARANCE
STOMACH RELIEF
bismuth subsalicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-691 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (SALICYLIC ACID - UNII:O414PZ4LPZ, BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color pink Score no score Shape OVAL Size 16mm Flavor Imprint Code 44;346 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-691-40 1 in 1 CARTON 03/31/2021 1 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 03/31/2021 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(63868-691) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(63868-691) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(63868-691)