Label: CLINDAMYCIN HYDROCHLORIDE capsule, gelatin coated
- NDC Code(s): 69238-2121-3, 69238-2121-4
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride capsules, USP and other antibacterial drugs, clindamycin hydrochloride capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
BOXED WARNING
(What is this?)
WARNING
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin hydrochloride and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
Because clindamycin hydrochloride therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate, as described in the INDICATIONS AND USAGE section. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
DESCRIPTION
Clindamycin hydrochloride is the hydrated hydrochloride salt of clindamycin. Clindamycin is a semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent compound lincomycin.
Clindamycin hydrochloride capsules, USP contain clindamycin hydrochloride equivalent to 300 mg of clindamycin.
Inactive ingredients:Corn starch, lactose monohydrate and magnesium stearate. Capsule Shell and Printing Ink: black iron oxide, D&C Yellow #10 Aluminum Lake, FD&C Blue #1, FD&C Blue #1 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, gelatin, propylene glycol, shellac, and titanium dioxide.
The structural formula is represented below:
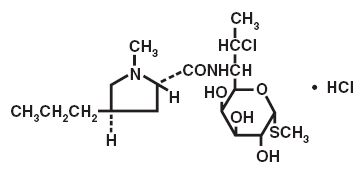
Molecular Formula: C18H33ClN2O5S·HCl
Molecular Weight: 461.44
The chemical name for clindamycin hydrochloride is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside monohydrochloride.
-
CLINICAL PHARMACOLOGY
Human Pharmacology
Absorption
Pharmacokinetic studies with a 150 mg oral dose of clindamycin hydrochloride in 24 normal adult volunteers showed that clindamycin was rapidly absorbed after oral administration. An average peak serum concentration of 2.50 mcg/mL was reached in 45 minutes; serum concentrations averaged 1.51 mcg/mL at 3 hours and 0.70 mcg/mL at 6 hours. Absorption of an oral dose is virtually complete (90%), and the concomitant administration of food does not appreciably modify the serum concentrations; serum concentrations have been uniform and predictable from person to person and dose to dose. Pharmacokinetic studies following multiple doses of clindamycin hydrochloride for up to 14 days show no evidence of accumulation or altered metabolism of drug. Doses of up to 2 grams of clindamycin per day for 14 days have been well tolerated by healthy volunteers, except that the incidence of gastrointestinal side effects is greater with the higher doses.
Distribution
Concentrations of clindamycin in the serum increased linearly with increased dose. Serum concentrations exceed the MIC (minimum inhibitory concentration) for most indicated organisms for at least six hours following administration of the usually recommended doses. Clindamycin is widely distributed in body fluids and tissues (including bones). No significant concentrations of clindamycin are attained in the cerebrospinal fluid, even in the presence of inflamed meninges.
Metabolism
In vitro studies in human liver and intestinal microsomes indicated that clindamycin is predominantly metabolized by Cytochrome P450 3A4 (CYP3A4), with minor contribution from CYP3A5, to form clindamycin sulfoxide and a minor metabolite, N-desmethylclindamycin.
Excretion
The average biological half-life is 2.4 hours. Approximately 10% of the bioactivity is excreted in the urine and 3.6% in the feces; the remainder is excreted as bioinactive metabolites.
Special Populations
Patients with Renal Impairment
Serum half-life of clindamycin is increased slightly in patients with markedly reduced renal function. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
Elderly Patients
Pharmacokinetic studies in elderly volunteers (61 to 79 years) and younger adults (18 to 39 years) indicate that age alone does not alter clindamycin pharmacokinetics (clearance, elimination half-life, volume of distribution, and area under the serum concentration-time curve) after IV administration of clindamycin phosphate. After oral administration of clindamycin hydrochloride, the average elimination half-life is increased to approximately 4.0 hours (range 3.4 to 5.1 h) in the elderly compared to 3.2 hours (range 2.1 to 4.2 h) in younger adults. The extent of absorption, however, is not different between age groups and no dosage alteration is necessary for the elderly with normal hepatic function and normal (age-adjusted) renal function1.
Obese Pediatric Patients Aged 2 to Less than 18 Years and Obese Adults Aged 18 to 20 Years
An analysis of pharmacokinetic data in obese pediatric patients aged 2 to less than 18 years and obese adults aged 18 to 20 years demonstrated that clindamycin clearance and volume of distribution, normalized by total body weight, are comparable regardless of obesity.
Microbiology
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is bacteriostatic.
Resistance
Resistance to clindamycin is most often caused by modification of specific bases of the 23S ribosomal RNA. Cross-resistance between clindamycin and lincomycin is complete. Because the binding sites for these antibacterial drugs overlap, cross-resistance is sometimes observed among lincosamides, macrolides and streptogramin B. Macrolide-inducible resistance to clindamycin occurs in some isolates of macrolide-resistant bacteria. Macrolide-resistant isolates of staphylococci and beta-hemolytic streptococci should be screened for induction of clindamycin resistance using the D-zone test.
Antimicrobial Activity
Clindamycin has been shown to be active against most of the isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)]:
Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenes
Anaerobic bacteria
Clostridium perfringens
Fusobacterium necrophorum
Fusobacterium nucleatum
Peptostreptococcus anaerobius
Prevotella melaninogenica
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for clindamycin against isolates of a similar genus or organism group. However, the efficacy of clindamycin in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria
Staphylococcus epidermidis (methicillin-susceptible strains)
Streptococcus agalactiae
Streptococcus anginosus
Streptococcus mitis
Streptococcus oralis
Anaerobic bacteria
Actinomyces israelii
Clostridium clostridioforme
Eggerthella lenta
Finegoldia (Peptostreptococcus) magna
Micromonas (Peptostreptococcus) micros
Prevotella bivia
Prevotella intermedia
Propionibacterium acnes
-
INDICATIONS AND USAGE
Clindamycin is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria.
Clindamycin is also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of colitis, as described in the BOXED WARNING, before selecting clindamycin, the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
Anaerobes: Serious respiratory tract infections such as empyema, anaerobic pneumonitis, and lung abscess; serious skin and soft tissue infections; septicemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, nongonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection.
Streptococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Staphylococci: Serious respiratory tract infections; serious skin and soft tissue infections.
Pneumococci: Serious respiratory tract infections.
Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clindamycin hydrochloride capsules and other antibacterial drugs, clindamycin hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
See BOXED WARNING
Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin hydrochloride, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Anaphylactic and Severe Hypersensitivity Reactions
Anaphylactic shock and anaphylactic reactions have been reported (see ADVERSE REACTIONS).
Severe hypersensitivity reactions, including severe skin reactions such as toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson syndrome (SJS), some with fatal outcome, have been reported (see ADVERSE REACTIONS).
In case of such an anaphylactic or severe hypersensitivity reaction, discontinue treatment permanently and institute appropriate therapy.
A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
Usage in Meningitis - Since clindamycin does not diffuse adequately into the cerebrospinal fluid, the drug should not be used in the treatment of meningitis.
-
PRECAUTIONS
General
Review of experience to date suggests that a subgroup of older patients with associated severe illness may tolerate diarrhea less well. When clindamycin is indicated in these patients, they should be carefully monitored for change in bowel frequency.
Clindamycin hydrochloride should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Clindamycin hydrochloride should be prescribed with caution in atopic individuals.
Indicated surgical procedures should be performed in conjunction with antibiotic therapy.
The use of clindamycin hydrochloride occasionally results in overgrowth of nonsusceptible organisms–particularly yeasts. Should superinfections occur, appropriate measures should be taken as indicated by the clinical situation.
Clindamycin dosage modification may not be necessary in patients with renal disease. In patients with moderate to severe liver disease, prolongation of clindamycin half-life has been found. However, it was postulated from studies that when given every eight hours, accumulation should rarely occur. Therefore, dosage modification in patients with liver disease may not be necessary. However, periodic liver enzyme determinations should be made when treating patients with severe liver disease.
Prescribing clindamycin hydrochloride capsules, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs, including clindamycin hydrochloride capsules, USP, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clindamycin hydrochloride capsules, USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clindamycin hydrochloride capsules, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
During prolonged therapy, periodic liver and kidney function tests and blood counts should be performed.
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Clindamycin is metabolized predominantly by CYP3A4, and to a lesser extent by CYP3A5, to the major metabolite clindamycin sulfoxide and minor metabolite N-desmethylclindamycin. Therefore inhibitors of CYP3A4 and CYP3A5 may increase plasma concentrations of clindamycin and inducers of these isoenzymes may reduce plasma concentrations of clindamycin. In the presence of strong CYP3A4 inhibitors, monitor for adverse reactions. In the presence of strong CYP3A4 inducers such as rifampicin, monitor for loss of effectiveness.
In vitro studies indicate that clindamycin does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2E1 or CYP2D6 and only moderately inhibits CYP3A4.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Fertility studies in rats treated orally with up to 300 mg/kg/day (approximately 1.6 times the highest recommended adult human dose based on mg/m2) revealed no effects on fertility or mating ability.
Pregnancy:
Teratogenic effects
In clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of congenital abnormalities.
Clindamycin should be used during the first trimester of pregnancy only if clearly needed. There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
Reproduction studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (3.2 and 1.6 times the highest recommended adult human dose based on mg/m2, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (1.3 and 0.7 times the highest recommended adult human dose based on mg/m2, respectively) revealed no evidence of teratogenicity.
Nursing Mothers
Limited published data based on breast milk sampling reports that clindamycin appears in human breast milk in the range of less than 0.5 to 3.8 mcg/mL. Clindamycin has the potential to cause adverse effects on the breast-fed infant's gastrointestinal flora. If oral or intravenous clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the breast-fed infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Pediatric Use
When clindamycin hydrochloride is administered to the pediatric population (birth to 16 years), appropriate monitoring of organ system functions is desirable.
Geriatric Use
Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients. However, other reported clinical experience indicates that antibiotic-associated colitis and diarrhea (due to Clostridium difficile) seen in association with most antibiotics occur more frequently in the elderly (> 60 years) and may be more severe. These patients should be carefully monitored for the development of diarrhea.
Pharmacokinetic studies with clindamycin have shown no clinically important differences between young and elderly subjects with normal hepatic function and normal (age-adjusted) renal function after oral or intravenous administration.
-
ADVERSE REACTIONS
The following reactions have been reported with the use of clindamycin.
Infections and Infestations: Clostridium difficile colitis
Gastrointestinal: Abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting, and diarrhea (see BOXED WARNING). The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS). Esophageal ulcer has been reported. An unpleasant or metallic taste has been reported after oral administration.
Hypersensitivity Reactions: Generalized mild to moderate morbilliform-like (maculopapular) skin rashes are the most frequently reported adverse reactions. Vesiculobullous rashes, as well as urticaria, have been observed during drug therapy. Severe skin reactions such as Toxic Epidermal Necrolysis, some with fatal outcome, have been reported (see WARNINGS). Cases of Acute Generalized Exanthematous Pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, anaphylactic shock, anaphylactic reaction and hypersensitivity have also been reported.
Skin and Mucous Membranes: Pruritus, vaginitis, angioedema and rare instances of exfoliative dermatitis have been reported (see Hypersensitivity Reactions.)
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Renal: Although no direct relationship of clindamycin to renal damage has been established, renal dysfunction as evidenced by azotemia, oliguria, and/or proteinuria has been observed.
Hematopoietic: Transient neutropenia (leukopenia) and eosinophilia have been reported. Reports of agranulocytosis and thrombocytopenia have been made. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of the foregoing.
Immune System: Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
Musculoskeletal: Cases of polyarthritis have been reported.
-
OVERDOSAGE
Significant mortality was observed in mice at an intravenous dose of 855 mg/kg and in rats at an oral or subcutaneous dose of approximately 2618 mg/kg. In the mice, convulsions and depression were observed.
Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
-
DOSAGE AND ADMINISTRATION
If significant diarrhea occurs during therapy, this antibiotic should be discontinued (see BOXED WARNING).
Adults: Serious infections - 150 to 300 mg every 6 hours. More severe infections - 300 to 450 mg every 6 hours.
Pediatric Patients (for children who are able to swallow capsules): Serious infections – 8 to 16 mg/kg/day (4 to 8 mg/lb/day) divided into three or four equal doses. More severe infections – 16 to 20 mg/kg/day (8 to 10 mg/lb/day) divided into three or four equal doses. Clindamycin should be dosed based on total body weight regardless of obesity.
To avoid the possibility of esophageal irritation, clindamycin hydrochloride capsules should be taken with a full glass of water.
Clindamycin hydrochloride capsules are not suitable for children who are unable to swallow them whole. The capsules do not provide exact mg/kg doses therefore it may be necessary to use the clindamycin palmitate oral solution in some cases.
Serious infections due to anaerobic bacteria are usually treated with clindamycin injection. However, in clinically appropriate circumstances, the physician may elect to initiate treatment or continue treatment with clindamycin hydrochloride capsules.
In cases of β-hemolytic streptococcal infections, treatment should continue for at least 10 days.
-
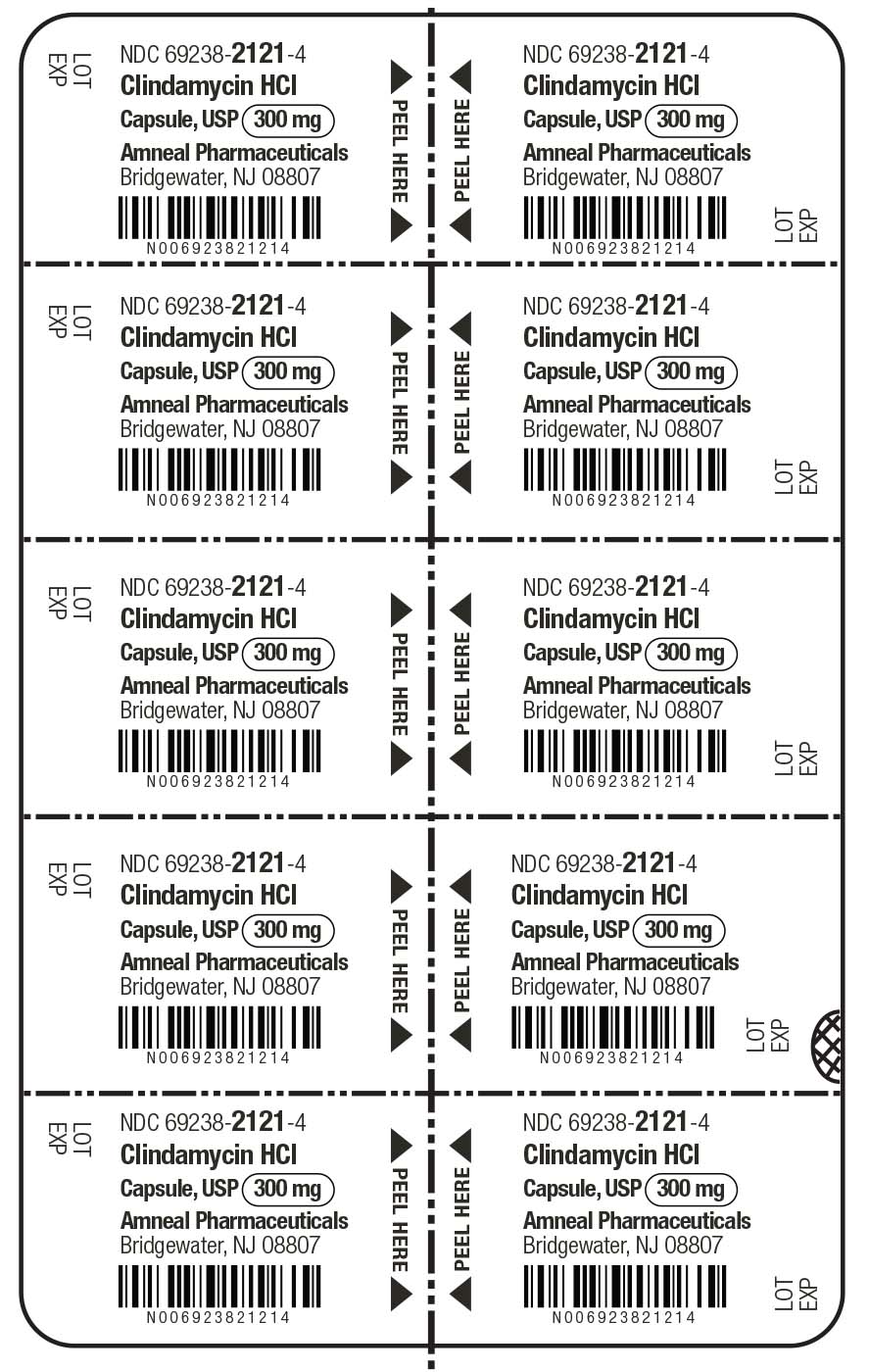
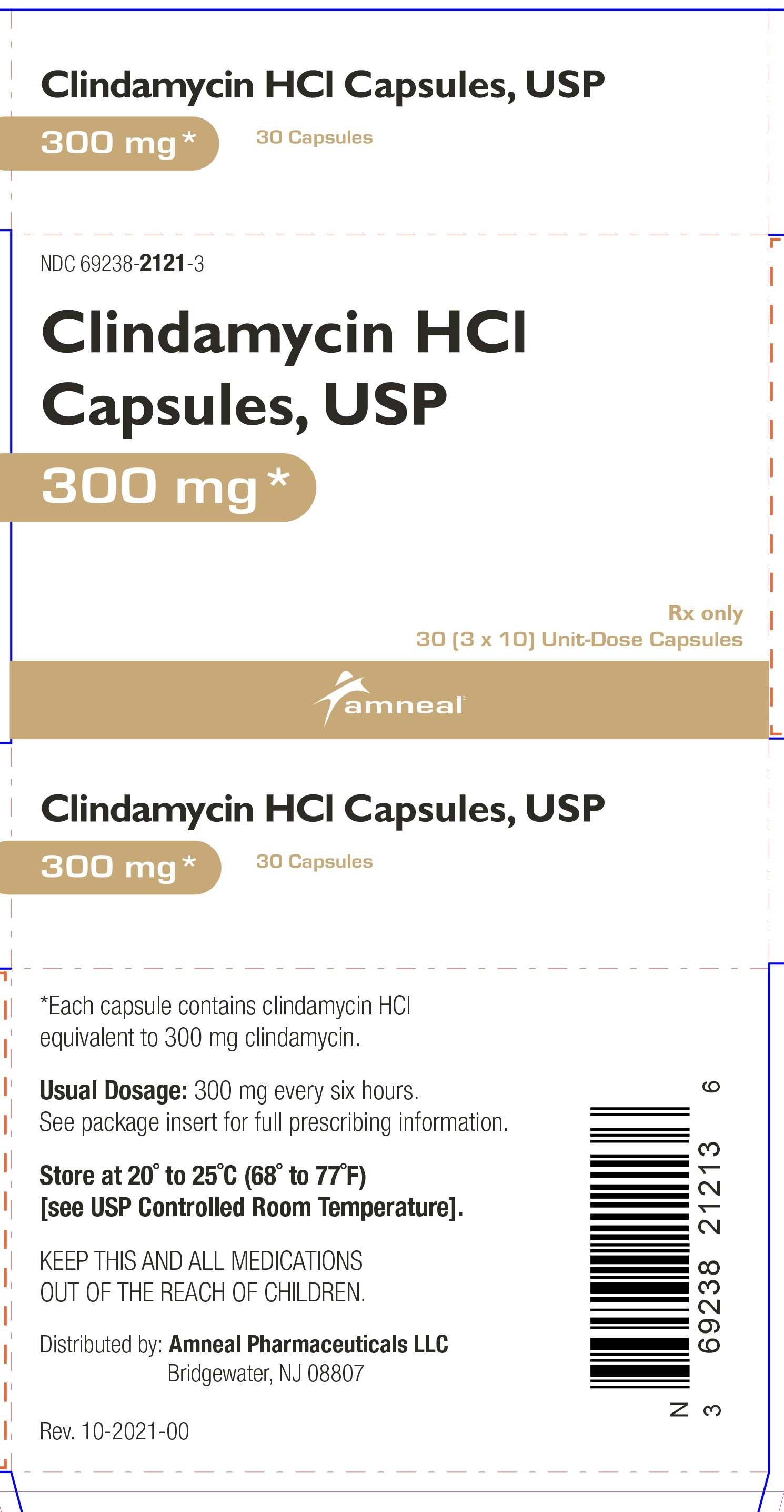
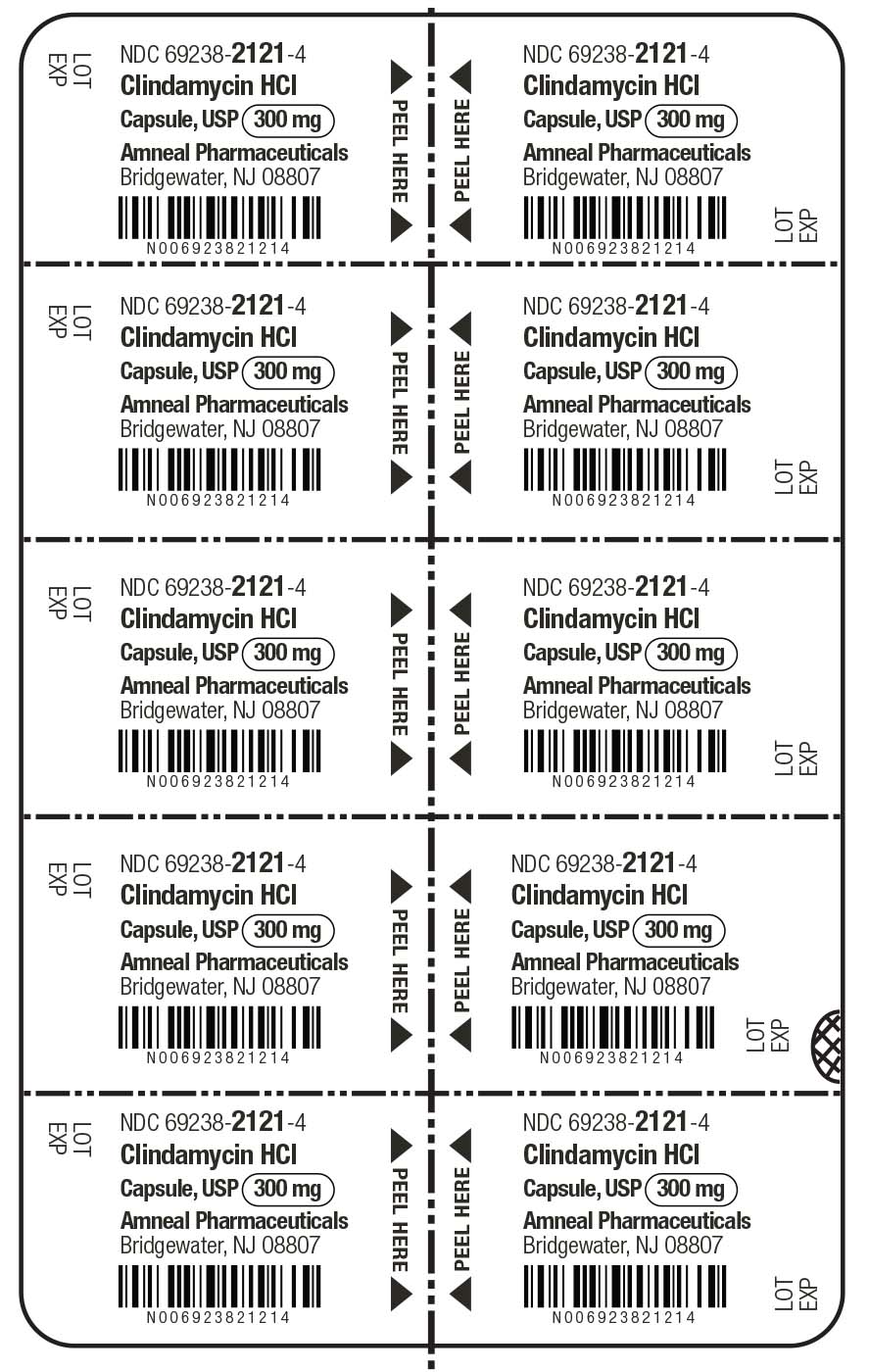
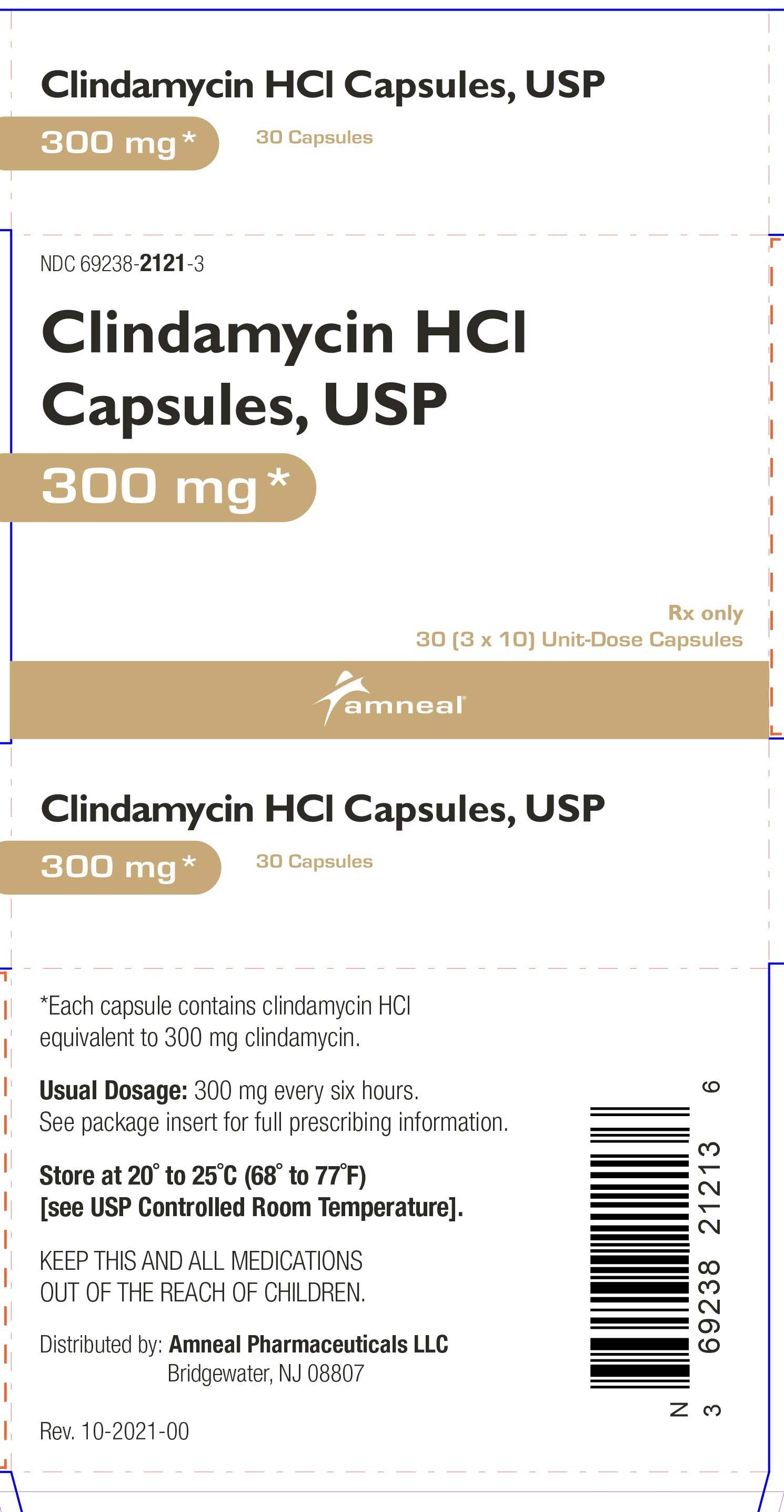
HOW SUPPLIED
Clindamycin hydrochloride capsules, USP contain clindamycin hydrochloride equivalent to 300 mg of clindamycin and are available as:
300 mg: light blue opaque cap imprinted with “CP” and light blue opaque body, imprinted with “5256” with black ink in cartons of 30 (3 blisters of 10 capsules each per carton) (NDC 69238-2121-3).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Rx only
- REFERENCES
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 300 mg
-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN HYDROCHLORIDE
clindamycin hydrochloride capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69238-2121 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN HYDROCHLORIDE (UNII: T20OQ1YN1W) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 300 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color blue (light blue opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code CP;5256 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69238-2121-3 3 in 1 CARTON 04/25/2022 1 NDC:69238-2121-4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065194 04/25/2022 Labeler - Amneal Pharmaceuticals NY LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals of New York, LLC 123797875 analysis(69238-2121) , label(69238-2121) , manufacture(69238-2121) , pack(69238-2121)