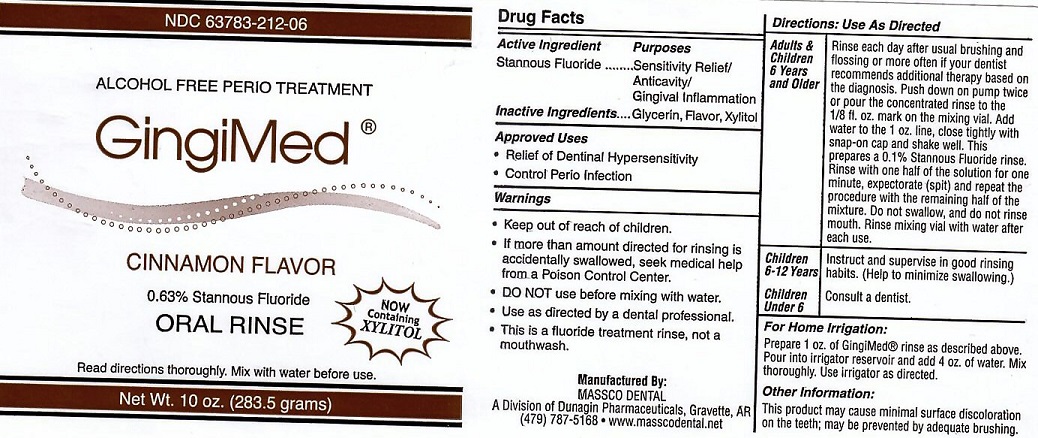
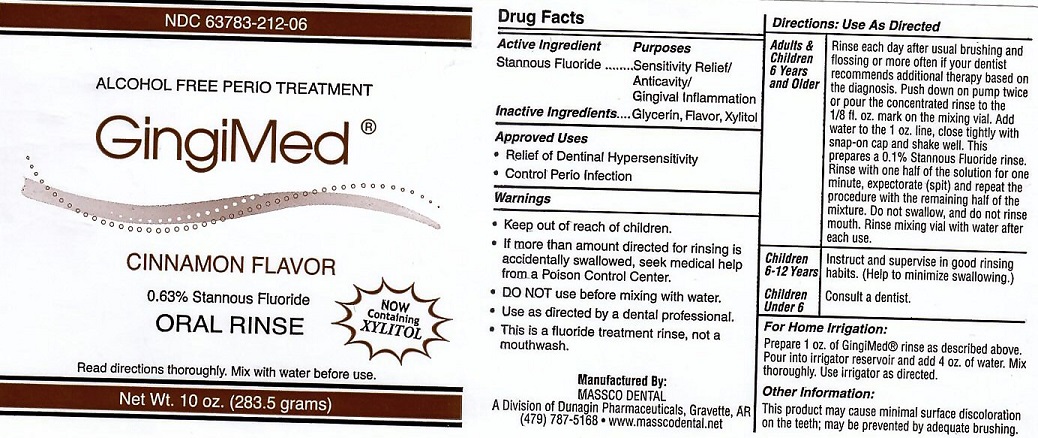
Label: GINGIMED- stannous fluoride liquid
- NDC Code(s): 63783-210-06, 63783-211-06, 63783-212-06
- Packager: Massco Dental
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS FOR USE
ADULTS AND CHILDREN 6 YEARS AND OLDER: RINSE EACH DAY AFTER USUAL BRUSHING AND FLOSSING OR MORE OFTEN IF YOUR DENTIST RECOMMENDS ADDITIONAL THERAPY BASED ON THE DIAGNOSIS. PUSH DOWN ON PUMP TWICE OR POUR THE CONCENTRATED RINSE TO THE 1/8 FL. OX. MARK ON THE MIXING VIAL. ADD WATER TO THE 1 OZ. LINE. CLOSE TIGHTLY WITH SNAP-ON CAP AND SHAKE WELL. THIS PREPARES A 0.1% STANNOUS FLUORIDE RINSE. RINSE WITH ONE HALF OF THE SOLUTION FOR ONE MINUTE, EXPECTORATE (SPIT) AND REPEAT THE PROCEDURE WITH THE REMAINING HALF OF THE MIXTURE. DO NOT SWALLOW AND DO NOT RINSE MOUTH. RINSE MIXING VIAL WITH WATER AFTER EACH USE.
CHILDREN 6-12 YEARS: INSTRUCT AND SUPERVISE IN GOOD RINSING HABITS. (HELP TO MINIMIZE SWALLOWING)
CHILDREN UNDER 6: CONSULT A DENTIST.
FOR HOME IRRIGATION: PREPARE 1 OZ. OF GINGIMED RINSE AS DESCRIBED ABOVE. POUR INTO IRRIGATOR RESIVOIR AND ADD 4 OZ. OF WATER. MIX THOROUGHLY. USE IRRIGATOR AS DESCRIBED
- OTHER INFORMATION
- WARNINGS
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
GINGIMED
stannous fluoride liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-210 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.19 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor SPEARMINT (MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-210-06 120 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 GINGIMED
stannous fluoride liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-211 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.19 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor GRAPE (CARIBBEAN ICE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-211-06 120 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 GINGIMED
stannous fluoride liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-212 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.19 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor CINNAMON (CINNAMON) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-212-06 120 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 Labeler - Massco Dental (008081858) Registrant - Massco Dental (008081858) Establishment Name Address ID/FEI Business Operations Massco Dental 008081858 manufacture(63783-210, 63783-211, 63783-212)