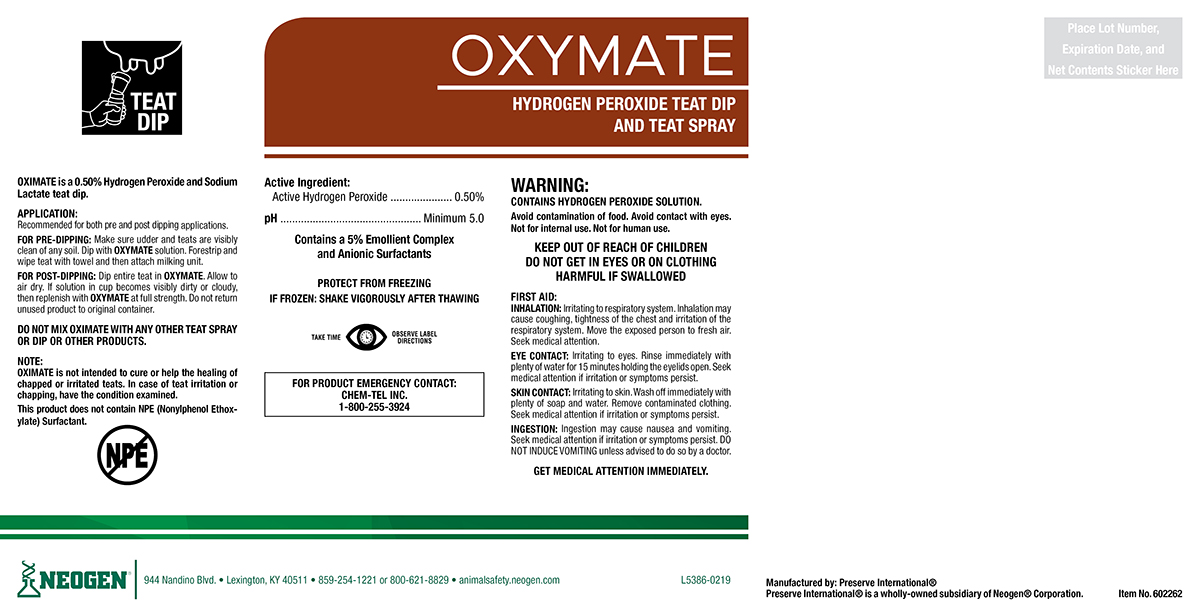
Label: OXYMATE- 0.5% hydrogen peroxide teat dip solution
-
NDC Code(s):
60648-9011-1,
60648-9011-2,
60648-9011-3,
60648-9011-4, view more60648-9011-5, 60648-9011-6, 60648-9011-7
- Packager: Preserve International
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
OXYMATE is a 0.5% hydrogen Peroxide and Sodium Lactate teat dip.
APPLICATION:
Recommended for both pre and post dipping applications.
FOR PRE-DIPPING: Make sure udder and teats are visibly clean of any soil. Dip with OXYMATE solution. Forestrip and wipe teat with towel and then attach milking unit.
FOR POST-DIPPING: Dip Entire teat in OXYMATE. Allow to air dry. If solution in cup becomes visibly dirty or cloudy, then replenish with OXYMATE at full strength. Do not return unused product to original container.
DO NOT MIX OXYMATE WITH ANY OTHER TEAT SPRAY OR DIP OR OTHER PRODUCTS.
NOTE:
OXYMATE is not intended to cure or help the healing of chapped or irritated teats. In case of teat irritation or chapping, have the condition examined.
This product does not contain NPE (Nonylphenol Ethoxylate) Surfactant.
-
WARNING:
CONTAINS HYDROGEN PEROXIDE SOLUTION
Avoid contamination of food. Avoid contact with eyes. Not for internal use. Not for human use.
KEEP OUT OF REACH OF CHILDREN
DO NOT GET IN EYES OR ON CLOTHING
HARMFUL IF SWALLOWED
FIRST AID:
INHALATION: Irritating to respiratory system. Inhalation may cause coughing, lightness of the chest and irritation of the respiratory system. Move the exposed person to fresh air. Seek medical attention.
EYE CONTACT: Irritating to eyes. Rinse immediately with plenty of water for 15 minutes holding the eyelids open. Seek medical attention if irritation or symptoms persist.
SKIN CONTACT: Irritating to skin. Wash off immediately with plenty of soap and water. Remove contaminated clothing. Seek medical attention if irritation or symptoms persist.
INGESTION: Ingestion may cause nausea and vomiting. Seek medical attention if irritation or symptoms persist. DO NOT INDUCE VOMITING unless advised to do so by a doctor.
GET MEDICAL ATTENTION IMMEDIATELY.FOR PRODUCT EMERGENCY CONTACT: Chem-Tel Inc. 1-800-255-3924
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OXYMATE
0.5% hydrogen peroxide teat dip solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:60648-9011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 5.95 g in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60648-9011-1 3.78 L in 1 JUG 2 NDC:60648-9011-2 18.9 L in 1 PAIL 3 NDC:60648-9011-3 56.7 L in 1 DRUM 4 NDC:60648-9011-4 113.4 L in 1 DRUM 5 NDC:60648-9011-5 207.9 L in 1 DRUM 6 NDC:60648-9011-6 945 L in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK 7 NDC:60648-9011-7 1039.5 L in 1 CONTAINER, FLEXIBLE INTERMEDIATE BULK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/18/2019 Labeler - Preserve International (808154199)