Label: PODOCON 25- podophyllum resin tincture
- NDC Code(s): 0574-0601-15
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Podocon-25® is composed of Podophyllin (Podophyllum Resin, American) 25% in Benzoin Tincture. Podophyllum Resin is the powdered mixture of resins removed from the May apple or Mandrake (PodophyllumpeltatumLinne’ ), a perennial plant of northern and middle United States(1). The podophyllin resin used in this product is exclusively the American podophyllin (rather than the Indian resin). American podophyllin typically has a reduced level of podophyllotoxin (see below).
-
CLINICAL PHARMACOLOGY:
Podophyllin is a cytotoxic agent that has been used topically in the treatment of genital warts. It arrests mitosis in metaphase, an effect it shares with other cytotoxic agents such as the vinca alkaloids(2). The active agent is podophyllotoxin, whose concentration varies with the type of podophyllin used; the American source normally containing one-fourth the amount of podophyllotoxin as the Indian source(3).
NOTE: PODOCON-25® IS TO BE APPLIED ONLY BY A PHYSICIAN. IT IS NOT TO BE DISPENSED TO THE PATIENT.
- INDICATIONS:
-
CONTRAINDICATIONS:
Podocon-25® is contraindicated in diabetics, patients using steroids or with poor blood circulation. Podocon-25® should not be used on bleeding warts, moles, birthmarks or unusual warts with hair growing from them. It is recommended that Podocon- 25® not be used during pregnancy (see Pregnancy warning below).
- WARNINGS:
- PRECAUTIONS:
-
ADVERSE REACTIONS:
The use of topical podophyllin has been known to result in paresthesia, polyneuritis, paralytic ileus, pyrexia, leukopenia, thrombocytopenia, coma and death(5).
Pregnancy:
There have been reports of complications associated with the topical use of podophyllin on condylomata of pregnant patients including birth defects, fetal death and stillbirth (6). In the absence of controlled safety studies, podophyllin remains contraindicated for use on pregnant patients.
-
DOSAGE AND ADMINISTRATION:
PODOCON-25® IS TO BE APPLIED ONLY BY A PHYSICIAN. IT IS NOT TO BE DISPENSED TO THE PATIENT. SHAKE WELL. Thoroughly cleanse affected area. Use supplied applicator to apply Podocon-25® sparingly to lesion. Avoid contact with healthy tissue. Allow to dry thoroughly. Only intact (no bleeding) lesions should be treated. As podophyllin is a powerful caustic and severe irritant, it is recommended the first application of Podocon-25® be left in contact for only a short time (30-40 minutes) to determine patient’s sensitivity. To avoid systemic absorption, time of contact should be minimum time necessary to produce the desired result (1 to 4 hours, depending on condition of lesion and of patient), the physician developing his/her own experience and technique. Large areas or numerous warts should not be treated at once.
After treatment time has elapsed, remove dried Podocon-25® thoroughly with alcohol or soap and water.
-
HOW SUPPLIED
Podocon-25® is available in 15-mL bottles with tapered tip applicator attached inside cap. NDC 0574-0601-15
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] in tight, light-resistant containers.
Rx Only
1) Blumgarten, A.F.: Text Book of Materia Medica, Pharmacology and Therapeutics; Ed. 7, New York, The Macmillan Company, 1937, pp. 220 and 223.
2) Green, L.K., Klima, M., Burns, T.; Arch Dermatol. Vol 124, Nov 1988, p. 1718.
3) Martindale, 28th Ed. London, 1982, pp. 1366, 1367.
4) Medical Letter; Vol 26, New Rochelle, N.Y., 1984, p10.
5) Fisher: Severe Systemic and Local Reactions to Topical Podophyllum Resins; Cutis, Volume 28, 1981.
6) Zackheim: Hazards of Topical Mitotic-Blocking Agents; Arch. Dermat. Volume 113, 1977.
Manufactured By
Perrigo®
Minneapolis, MN 55427
2124075
(05-12)
-
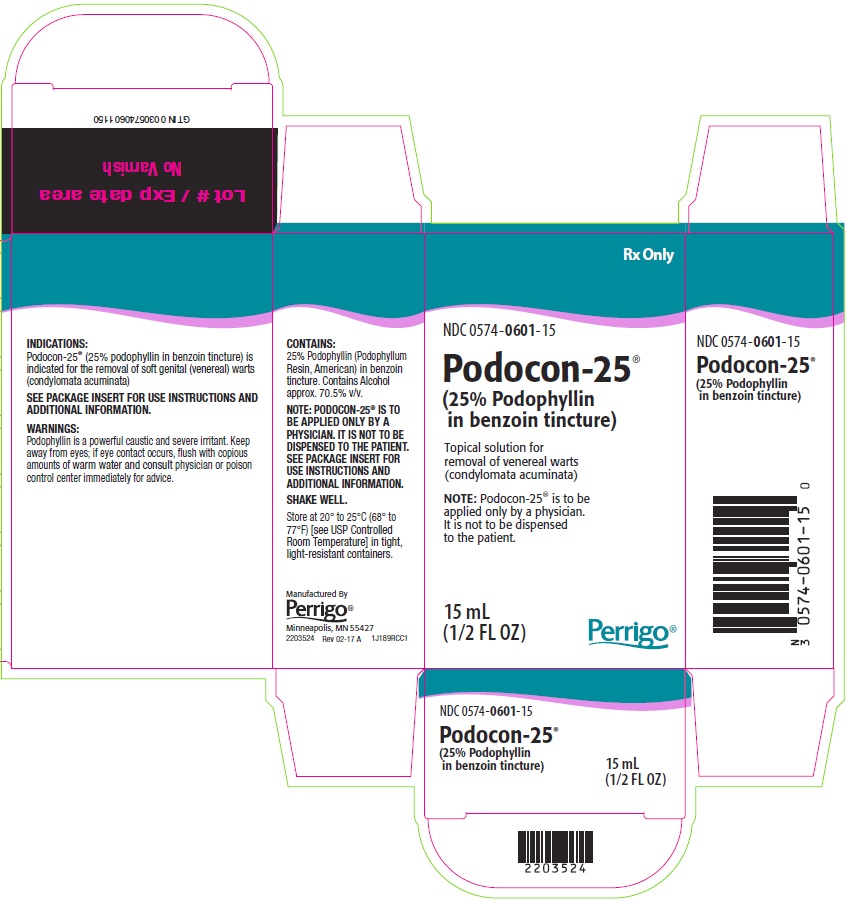
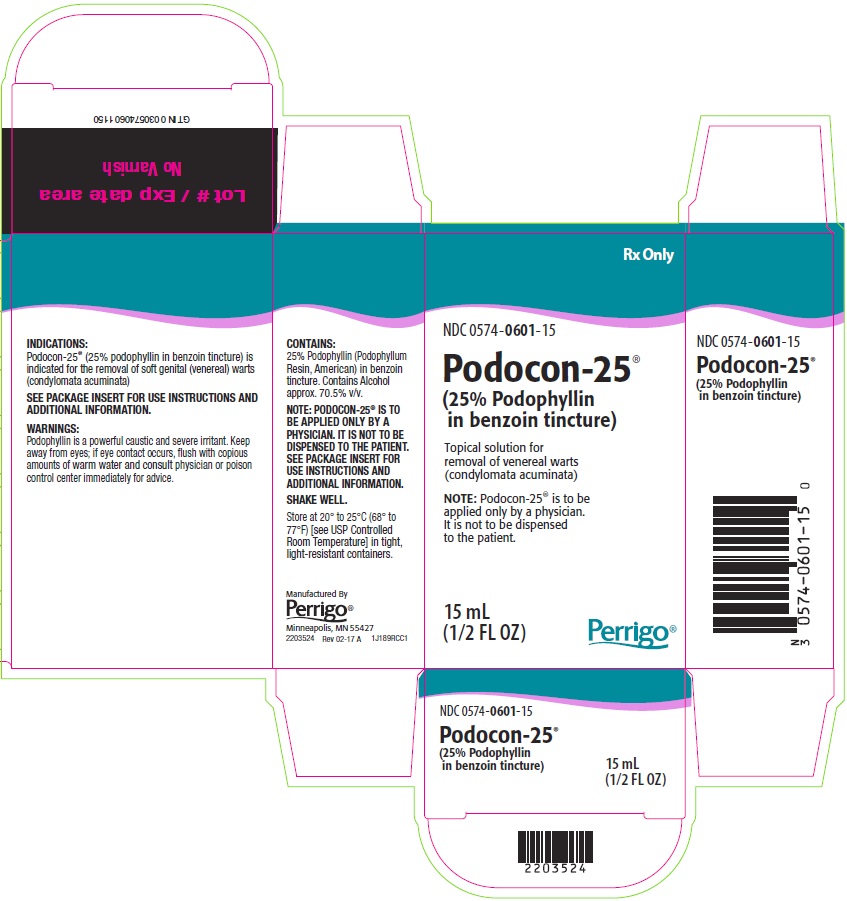
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
NDC 0574-0601-15
Podocon-25®
(25% Podophyllin in benzoin tincture)
Topical solution for removal of venereal warts (condylomata acuminata)
NOTE: Podocon-25® is to be applied only by a physician. It is not to be dispensed to the patient.
15 mL
(1/2 FL OZ)
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
PODOCON 25
podophyllum resin tinctureProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-0601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PODOPHYLLUM RESIN (UNII: 16902YVY2B) (PODOPHYLLUM RESIN - UNII:16902YVY2B) PODOPHYLLUM RESIN 1 mg in 4 mL Inactive Ingredients Ingredient Name Strength STYRAX BENZOIN RESIN (UNII: FE663Z8IRO) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-0601-15 1 in 1 CARTON 09/01/1990 1 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 09/01/1990 Labeler - Padagis US LLC (967694121)