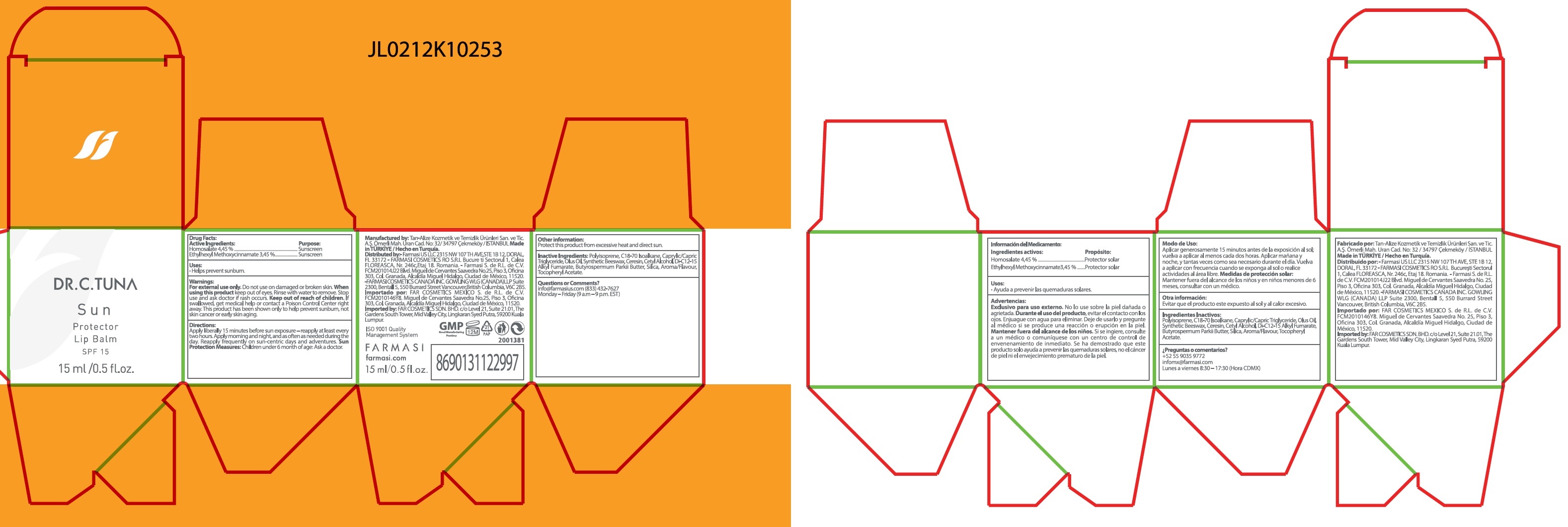
Label: DR C TUNA SUN PROTECTOR LIP BALM SPF 15- homosalate, octinoxate cream
- NDC Code(s): 74690-020-00
- Packager: Farmasi US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts:
- Active Ingredients:
- Uses:
- Warnings:
- Directions:
- Other information:
- Inactive Ingredients:
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DR C TUNA SUN PROTECTOR LIP BALM SPF 15
homosalate, octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74690-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 44.5 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 34.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) DI-C12-15 ALKYL FUMARATE (UNII: A1CB3Z898P) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74690-020-00 1 in 1 BOX 01/05/2022 1 15 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/05/2022 Labeler - Farmasi US LLC (113303351)