Label: DENTEK CANKER COVER- menthol patch, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 60630-091-01, 60630-091-02 - Packager: DenTek Oral Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 22, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (per each patch)
- Purpose
- Uses
- Warnings
-
Directions
-
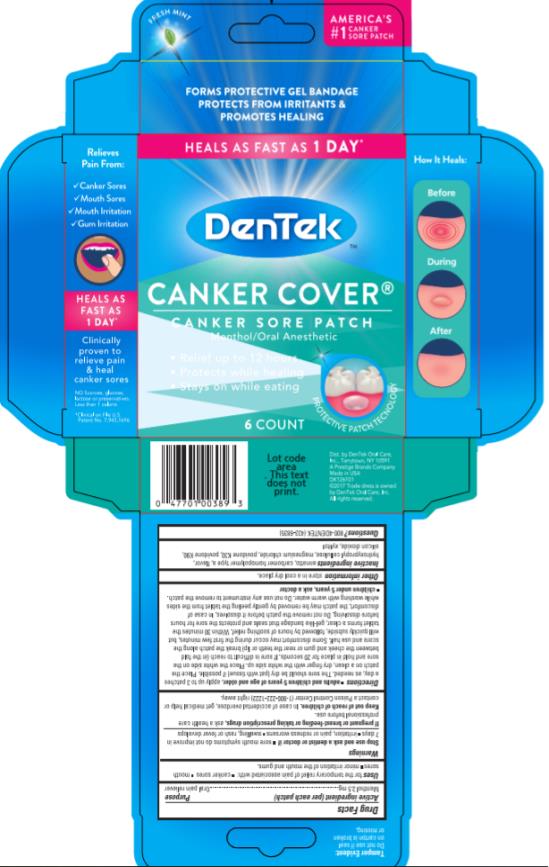
adults and children 5 years of age and older, apply up to 3 times a day, as needed
The sore should be dry (pat with tissue) if possible. Place the patch on a clean, dry finger with the white side up. Place the white side on the sore and hold in place for 20 seconds. If sore is difficult to reach (in the fold between the cheek and gum or near the teeth or lip) break the patch along the score and use half. Some discomfort may occur during the first few minutes, but will quickly subside, followed by hours of soothing relief. Within 30 minutes the tablet forms a clear, gel-like bandage that seals and protects the sore for hours before dissolving. Do not remove the patch before it dissolves. In case of discomfort, the patch may be removed by gently peeling the tablet from the sides while washing with warm water. Do not use any instrument to remove the patch.
- children under 5 years, ask a doctor
-
adults and children 5 years of age and older, apply up to 3 times a day, as needed
- Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTEK CANKER COVER
menthol patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60630-091 Route of Administration TRANSMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2.5 mg in 194.85 mg Inactive Ingredients Ingredient Name Strength ANNATTO (UNII: 6PQP1V1B6O) CARBOMER HOMOPOLYMER TYPE A (UNII: F68VH75CJC) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POVIDONE K30 (UNII: U725QWY32X) POVIDONE K90 (UNII: RDH86HJV5Z) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60630-091-02 6 in 1 CARTON 11/11/2015 1 NDC:60630-091-01 2.5 mg in 1 DOSE PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 11/11/2015 Labeler - DenTek Oral Care, Inc. (153818646)