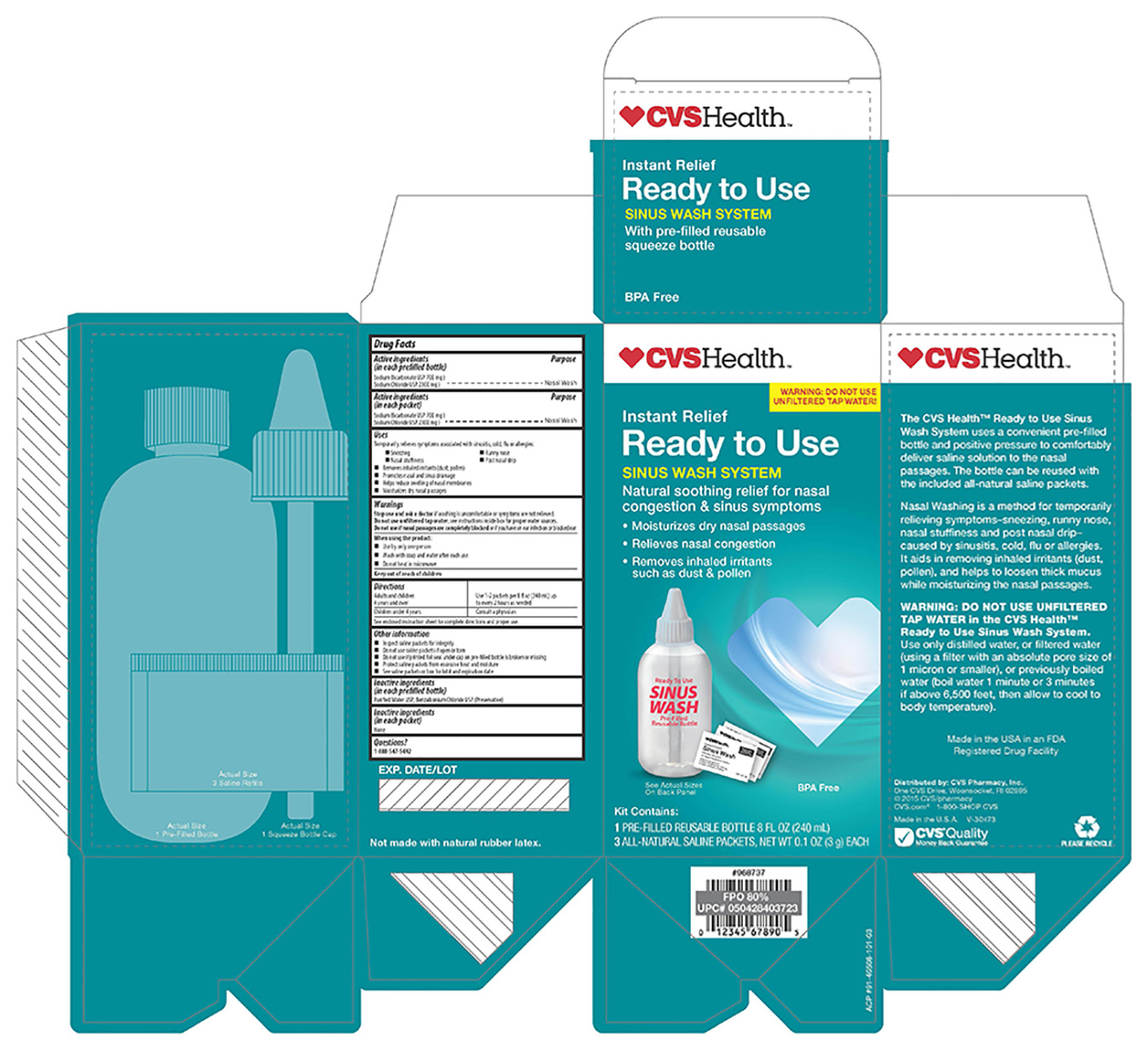
Label: CVS- sodium chloride, sodium bicarbonate kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-408-01, 59779-865-00, 59779-867-03 - Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 25, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Stop use and ask a doctor if washing is uncomfortable or symptoms are not relieved.
Do not use unfiltered tap water. see instructions inside box for proper water sources.
Do not use if nasal passages are completely blocked or if you have an ear infection or blocked ear.
When using this product:
-Use by only one person
-Wash with soap and water after each use
-Do not heat in microwave
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
INSTRUCTIONS FOR USE
Sinus Wash
Pre-Filled Ready to Use
Saline Sinus Wash Kit
Instructions
DO NOT
SINUS WASH
WITH UNFILTERED
TAP WATER.
See important note*
for proper water
sources.
Read through entire instruction sheet
before using for the fi rst time.
* IMPORTANT NOTE
Do not sinus wash with unfi ltered tap water. Use either distilled, or
sterile, or fi ltered (using a fi lter with an absolute pore size of 1 micron or
smaller) or previously boiled water (boil water 1 minute or 3 minutes if at
elevations above 6,500 feet, then allow to cool to body temperature).
Instructions for Use of the Ready to Use
Sinus Wash Kit (pre-filled with solution)
Read through entire section.
1. Wash your hands and remove cap and foil safety seal from the top of
the pre-filled bottle.
2. Assemble white cap and tube and then place on pre-filled bottle and
tighten securely.
3. Lean over the sink with your head bent down so you are looking directly
into the basin. Place the tip of the cap up to the right nostril and gently
insert the tip so that it forms a comfortable seal. Aim the tip at the back
of your head, not at the top of your head.
4. Squeeze the bottle gently so that the solution enters the right nostril. Do not
inhale or “snort” the solution into the nose – breathe through your mouth.
5. In a few moments, the solution will begin to drain out of the left
nostril. Continue to squeeze the bottle gently until you have used
approximately half of the solution.
6. Remove the tip of the bottle from your nostril, then exhale through both
nostrils to clear them of excess mucus and solution. Gently blow your
nose into a tissue.
7. Repeat the procedure on the left nostril with the remaining solution.
8. A small amount of solution will remain at the bottom of the bottle.
Discard the remaining solution.
9. Thoroughly rinse the bottle after each use with water that has been
either distilled, sterilized, filtered or previously boiled and leave the
bottle open to air dry completely.
10. Bottle, tube and cap may be reused with included packets. See
instructions that follow.
Isotonic Solution
Hypertonic Solution
1 Saline Packet per
8 fl oz. (240 mL)
2 Saline Packets per
8 fl oz. (240 mL)
Refill instructions for the Ready to Use
Sinus Wash Kit (with saline packets)
Read through entire section.
The Ready to Use Sinus Wash Kit can be used with the enclosed Saline
packets to make either an Isotonic or Hypertonic solution. Check with the
guide below or consult with your physician for best use.
An Isotonic solution creates a mild, less concentrated salt solution of 9 mg
of Sodium Chloride per mL of water, which has a similar salt concentration
of your body and may be more comfortable to use for some people initially
than a more concentrated Hypertonic solution.
A Hypertonic solution creates a higher, more concentrated salt solution
which is similar to the salt content of ocean water.
1. Wash your hands and make sure the bottle has been rinsed with water
that has been either distilled, sterilized, filtered or previously boiled.
2. Pour the pre-mixed saline dry ingredients into the Squeeze Bottle.
First-time users should start with 1 packet of the saline dry ingredients
to make an isotonic solution. As you become more accustomed to
the system, work up to using 2 full packets for a hypertonic solution.
Additional packets may be purchased from your nearest pharmacy.
3. Fill the bottle to the 8 oz line with proper water*.
4. Tighten the cap on the bottle securely, place one finger over the tip
of the cap and gently shake the bottle until the dry ingredients have
completely dissolved.
5. Lean over the sink with your head bent down so you are looking directly
into the basin. Place the tip of the cap up to the right nostril and gently
insert the tip so that it forms a comfortable seal. Aim the tip at the back
of your head, not at the top of your head. (continued on back)
Hints for a more comfortable nasal wash:
• Begin using the Sinus Wash System slowly – especially with children. A full
pot or bottle of solution on each side is not necessary to receive the full benefit.
• If the solution is too warm or too cold, the nasal wash will be uncomfortable.
• If stinging or irritation occurs, try using 1 packet of dry ingredients per
pot or bottle. Gradually work up to using the solution at full strength using 2
full packets per bottle. Do not use less than 1 packet.
• If you experience ear discomfort after use, try to blow your nose more gently
after the wash. If the problem persists, the openings of your Eustachian tubes
may be particularly wide and use of the system should be discontinued.
• Breathe continuously through the mouth when using the Sinus Wash System
to avoid solution draining from the back of the nose into the mouth.
• Some people experience irritation of the skin just inside the nostrils with
repeated nasal washing. Applying a thin layer of aloe to the inside of the
nostrils before or after the nasal wash will be soothing - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS
sodium chloride, sodium bicarbonate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-867 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-867-03 1 in 1 KIT 07/01/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 BOTTLE 3 g Part 2 1 PACKET 3000 mg Part 1 of 2 CVS
sodium bicarbonate, sodium chloride solutionProduct Information Item Code (Source) NDC:59779-865 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 22.2 mg in .1 g SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 77.8 mg in .1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC:59779-865-00 240 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/01/2016 Part 2 of 2 CVS
sodium bicarbonate, sodium chloride powderProduct Information Item Code (Source) NDC:59779-408 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 22.8 mg in 100 mg SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 77.8 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC:59779-408-01 3000 mg in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2014 Labeler - CVS Pharmacy (062312574)