Label: THERAPEUTIC- coal tar shampoo
- NDC Code(s): 59779-850-30
- Packager: CVS Pharmacy, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- Ask a doctor before use if you have
-
When using this product
- do not get into eyes. If contact occurrs, rinse eyes thoroughly with water
- use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application
- do not use for prolonged periods without consulting a doctor
- do not use with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor
- Stop use and ask a doctor if
- Keep out of reach of children.
- California Proposition 65 Warning
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
-
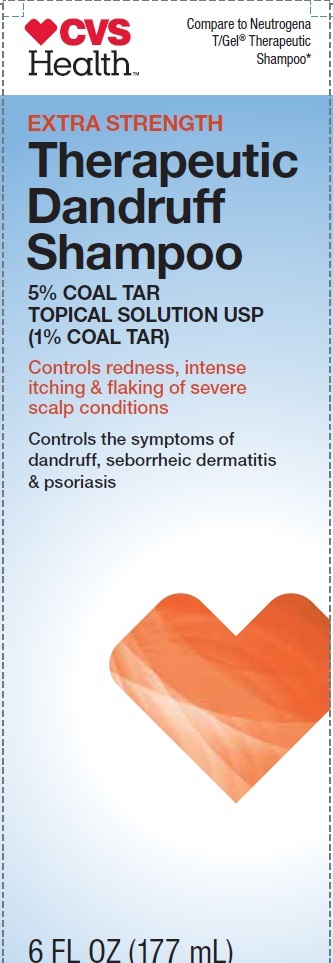
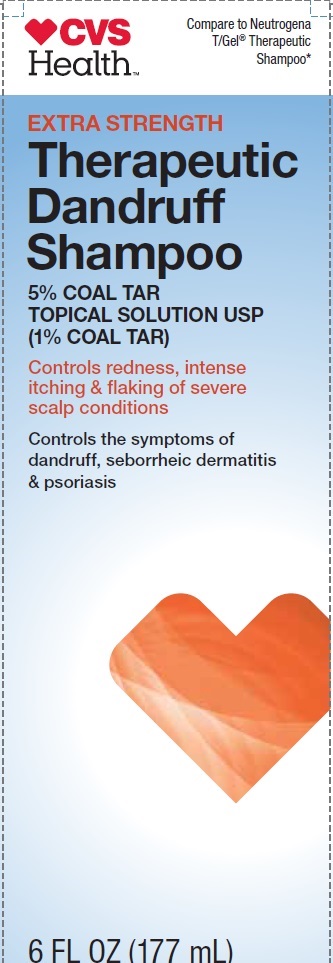
PRINCIPAL DISPLAY PANEL
CVS Health
Compare to Neutrogena T/Gel Therapeutic Shampoo*
EXTRA STRENGTH
Therapeutic Dandruff Shampoo
5% COAL TAR TOPICAL SOLUTION USP (1% COAL TAR)
Controls redness, intense itching & flaking of severe scalp conditions
Controls the symptoms of dandruff, sevorrheic dermatitis & psoriasis
6 FL OZ (177 mL)
-
INGREDIENTS AND APPEARANCE
THERAPEUTIC
coal tar shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAL TAR (UNII: R533ESO2EC) (COAL TAR - UNII:R533ESO2EC) COAL TAR 1.02 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLYSORBATE 20 (UNII: 7T1F30V5YH) LAURETH-4 (UNII: 6HQ855798J) BISHYDROXYETHYL DIHYDROXYPROPYL STEARAMMONIUM CHLORIDE (UNII: 32II08O72A) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) EDETATE SODIUM (UNII: MP1J8420LU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) IMIDUREA (UNII: M629807ATL) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CARAMEL (UNII: T9D99G2B1R) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-850-30 1 in 1 CARTON 03/15/2016 1 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 03/15/2016 Labeler - CVS Pharmacy, Inc. (062312574) Registrant - Vi-Jon, LLC (790752542) Establishment Name Address ID/FEI Business Operations Vi-Jon, LLC 790752542 manufacture(59779-850) Establishment Name Address ID/FEI Business Operations Vi-Jon, LLC 088520668 manufacture(59779-850)