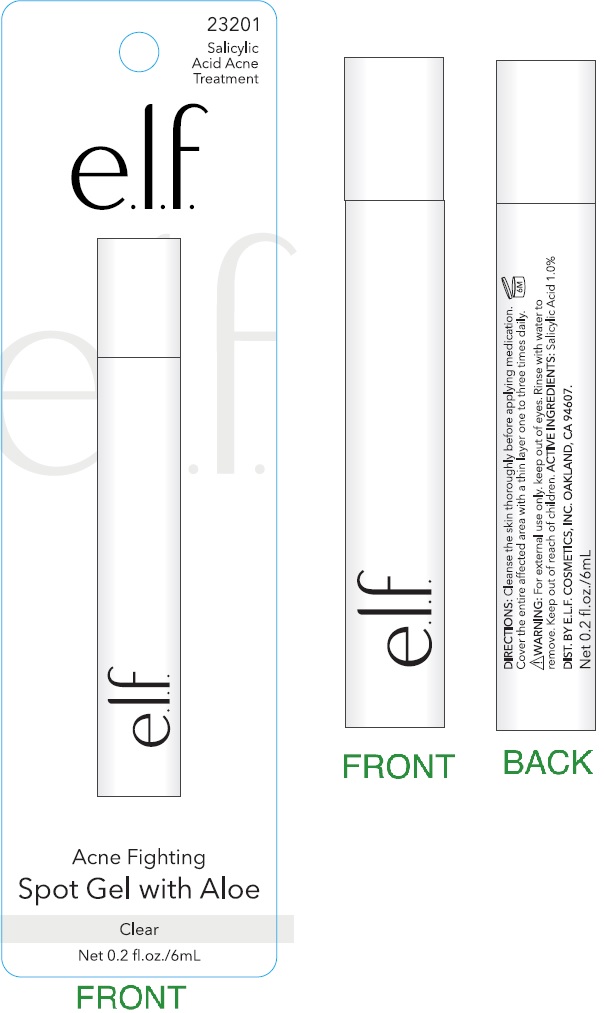
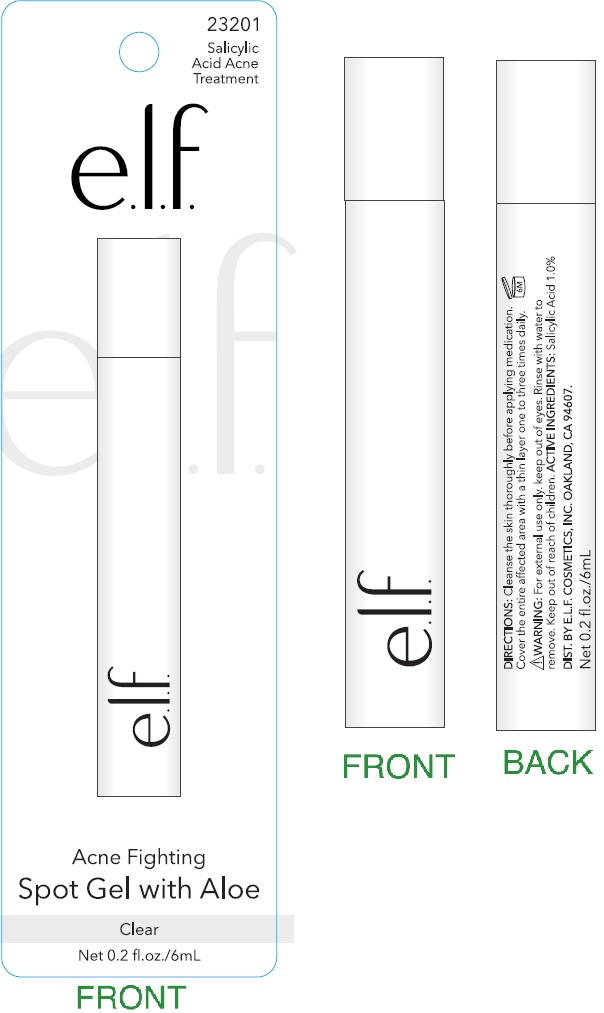
Label: ACNE FIGHTING SPOT WITH ALOE CLEAR- salicylic acid gel
- NDC Code(s): 70412-411-06
- Packager: Zhejiang Ayan Biotech Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- Cleanse the skin thoroughly before applying medication.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other Information
- Inactive ingredients
- Questions or comments
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ACNE FIGHTING SPOT WITH ALOE CLEAR
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70412-411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) LAURETH-7 (UNII: Z95S6G8201) TEA TREE OIL (UNII: VIF565UC2G) ALOE VERA LEAF (UNII: ZY81Z83H0X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70412-411-06 1 in 1 BOX 06/01/2018 1 6 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/01/2018 Labeler - Zhejiang Ayan Biotech Co.,Ltd. (544377996) Establishment Name Address ID/FEI Business Operations Zhejiang Ayan Biotech Co.,Ltd. 544377996 manufacture(70412-411)