Label: ISOTRETINOIN capsule
-
NDC Code(s):
70771-1557-4,
70771-1557-8,
70771-1558-4,
70771-1558-8, view more70771-1559-8, 70771-1560-4, 70771-1560-8
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- MEDICATION GUIDE
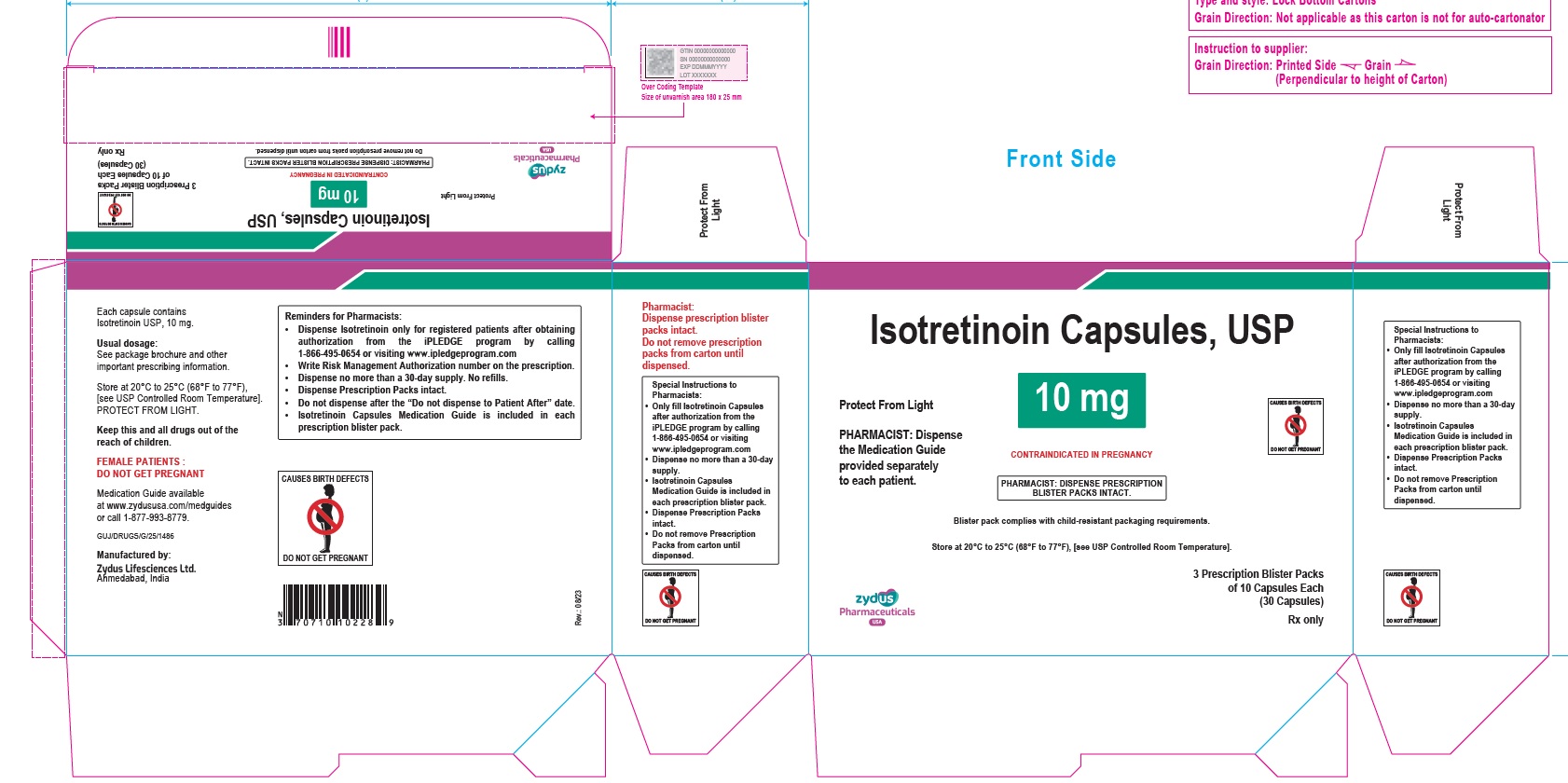
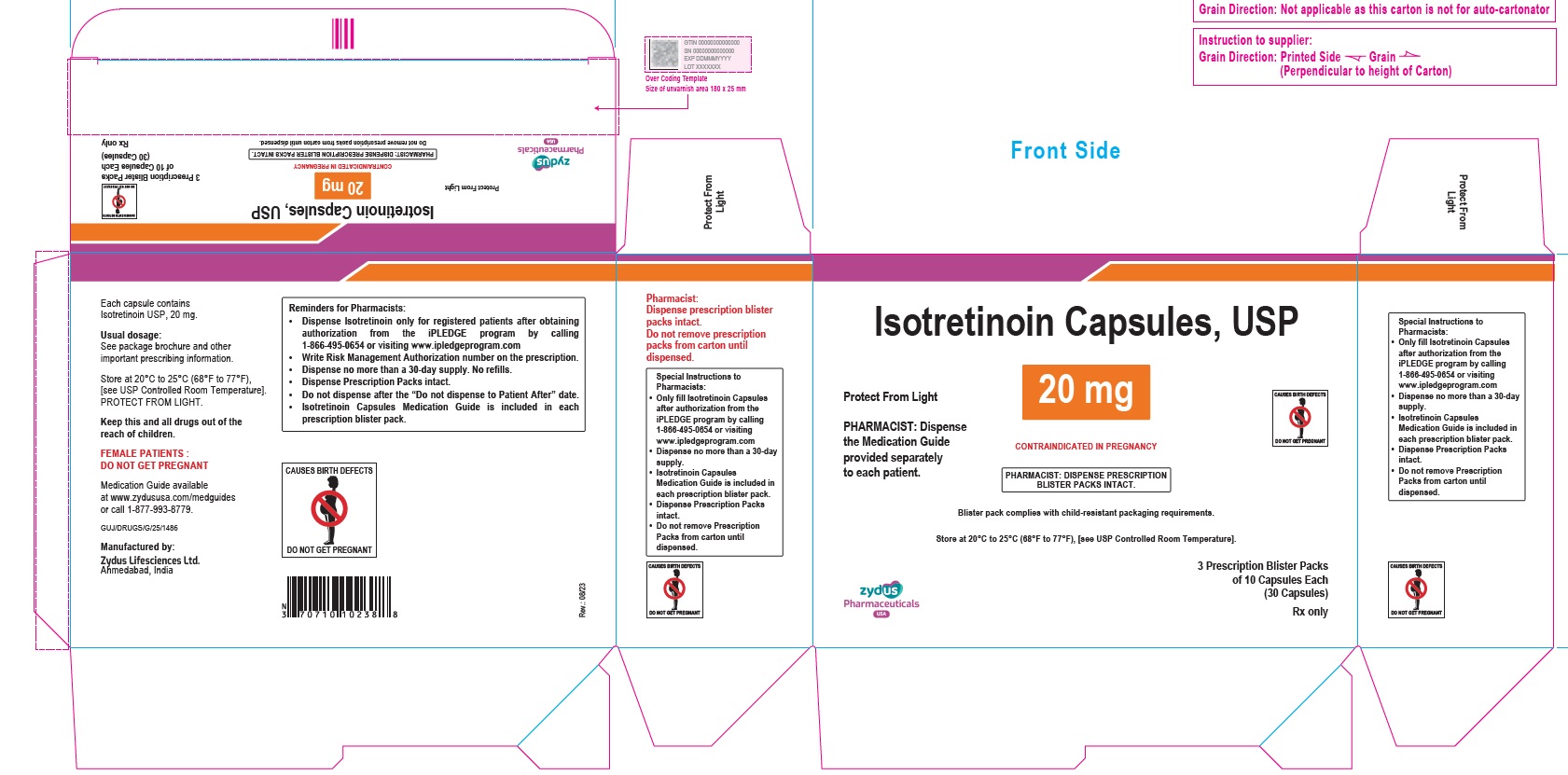
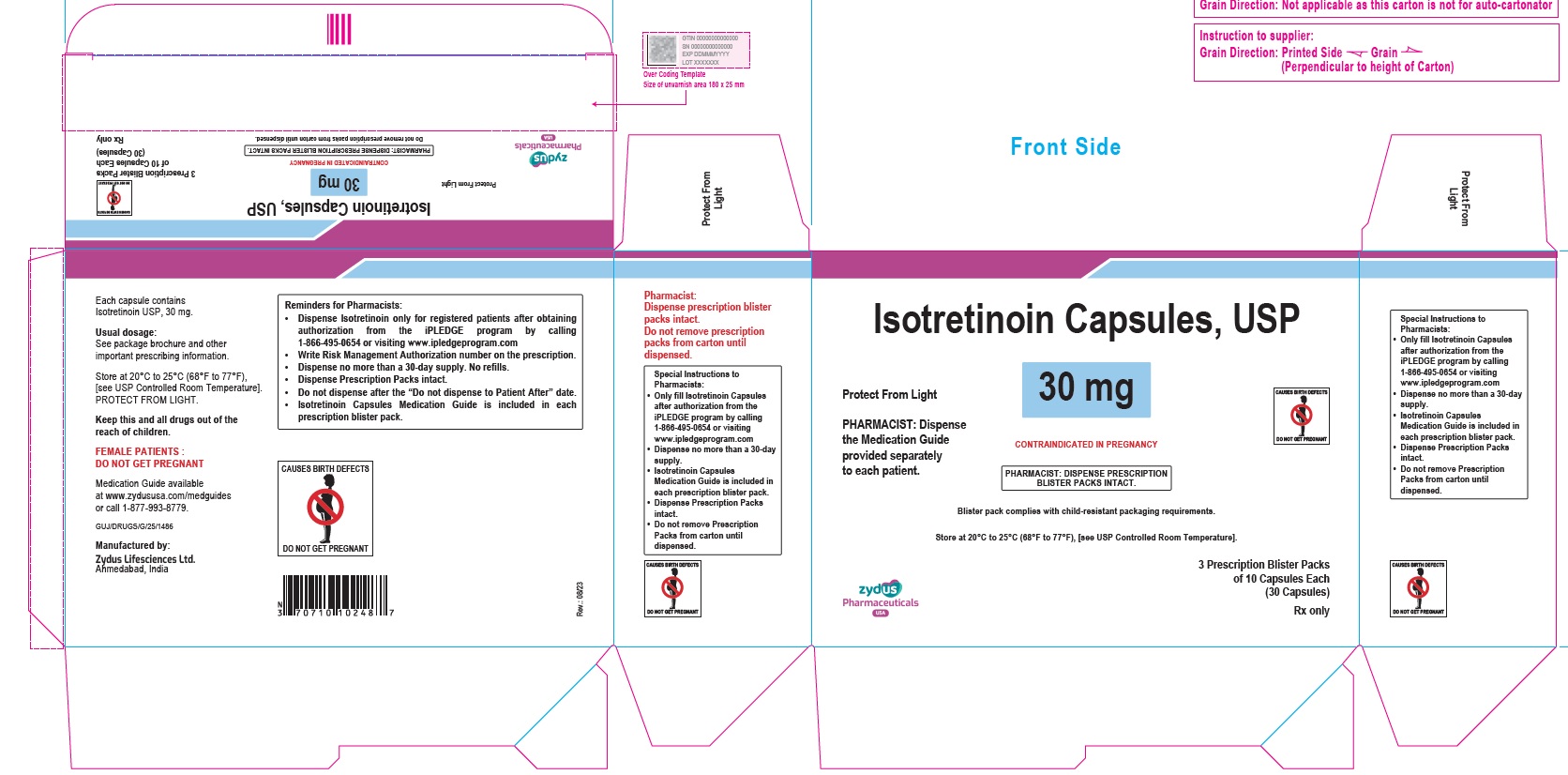
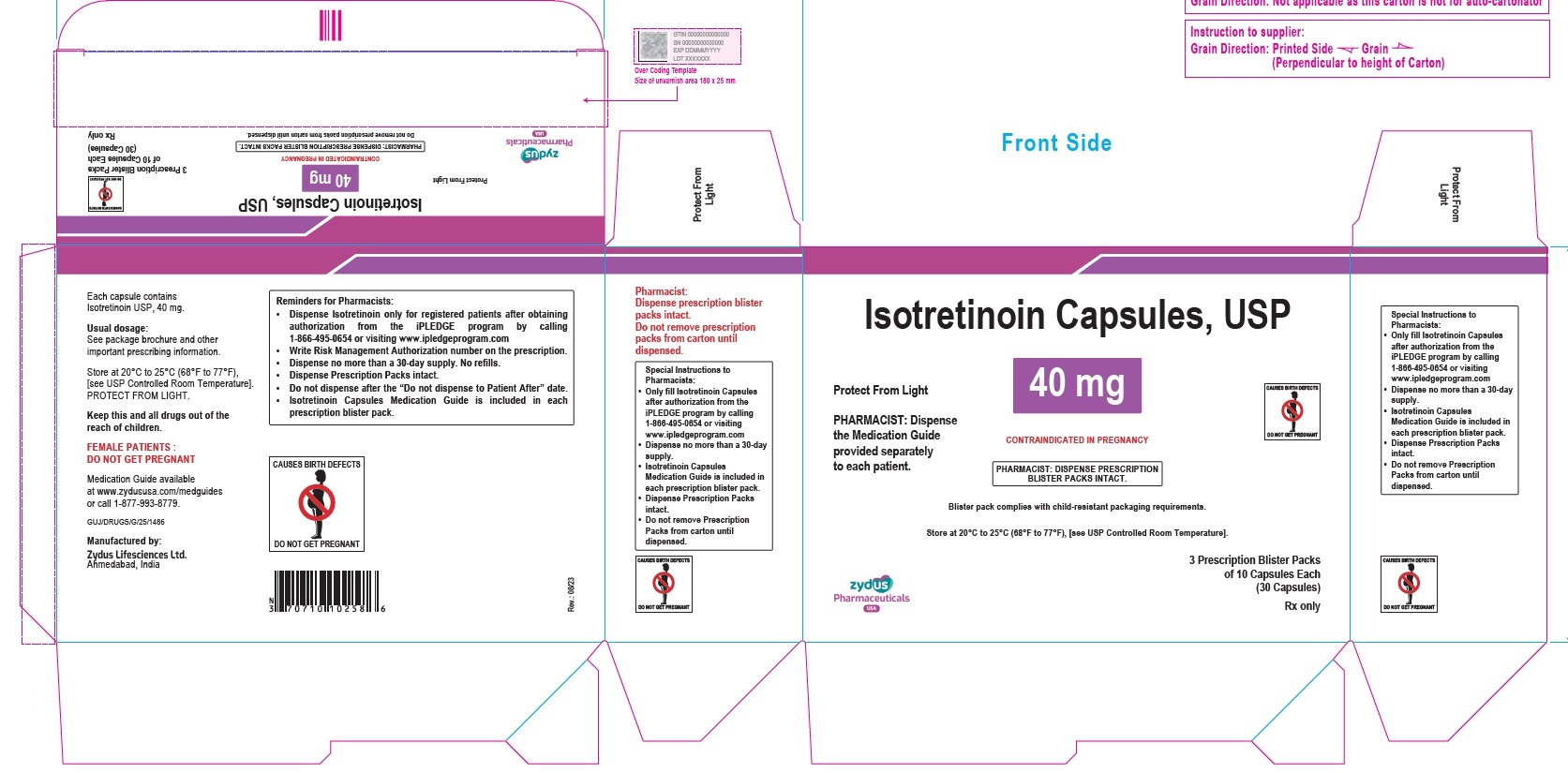
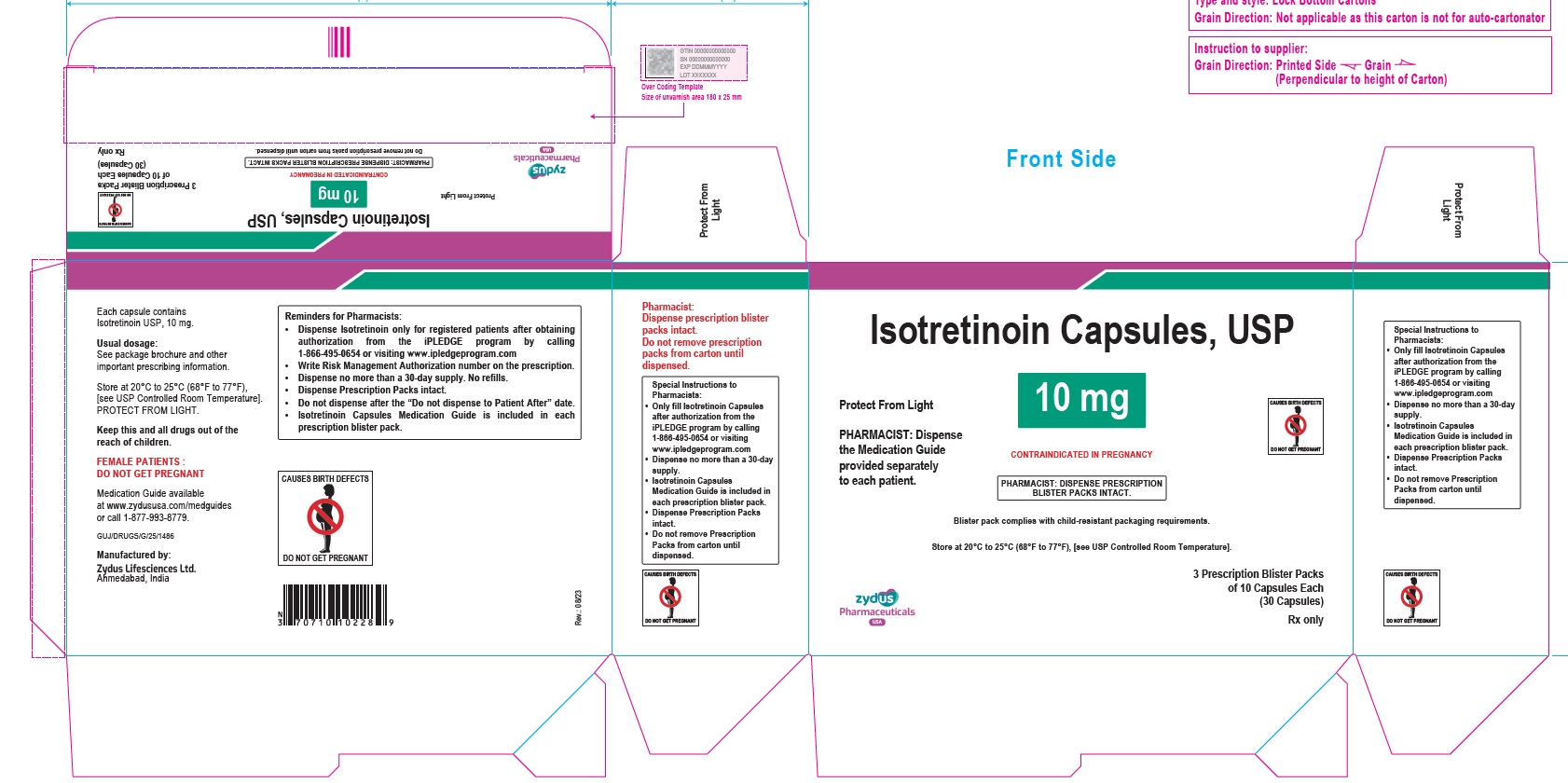
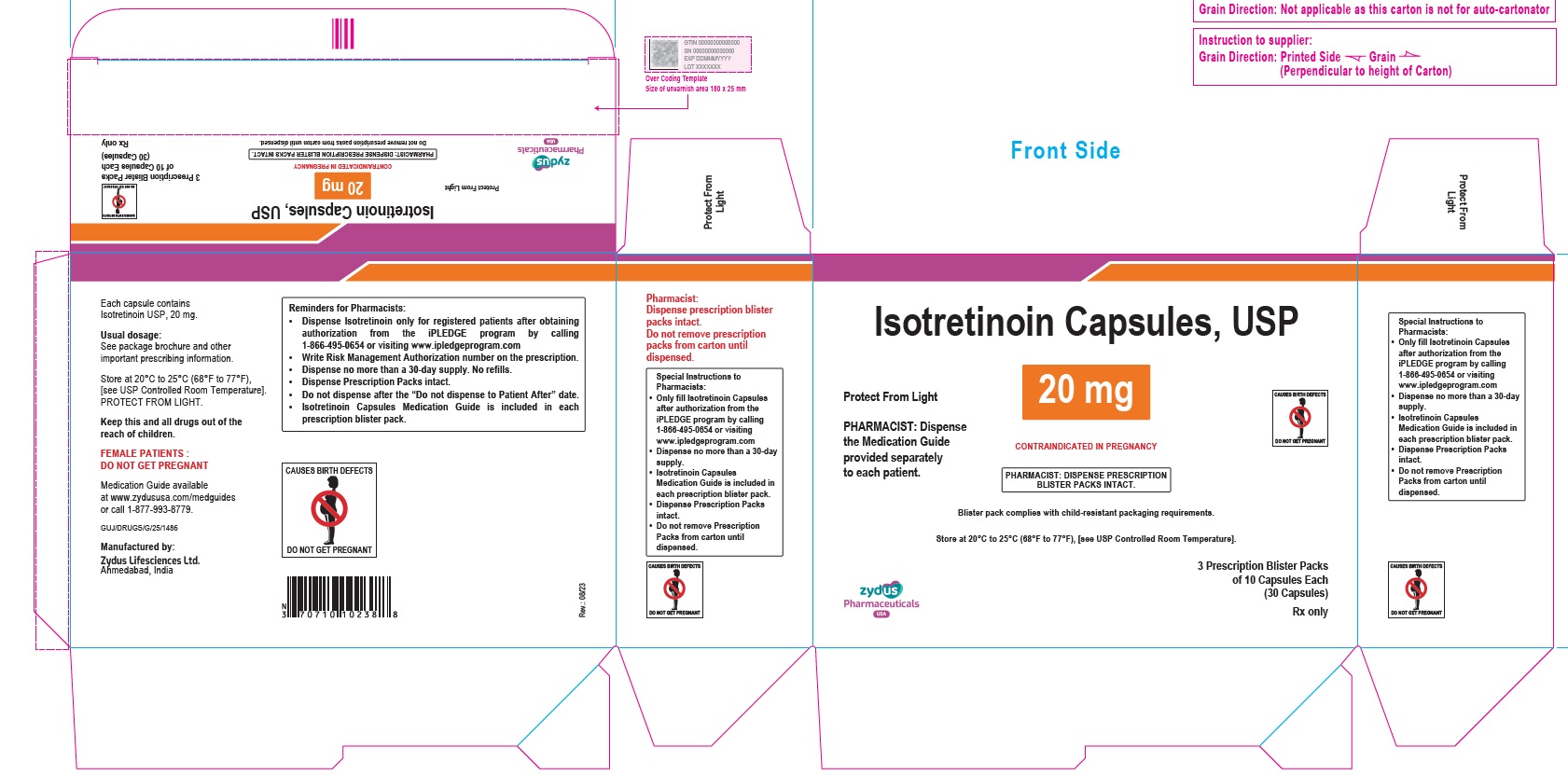
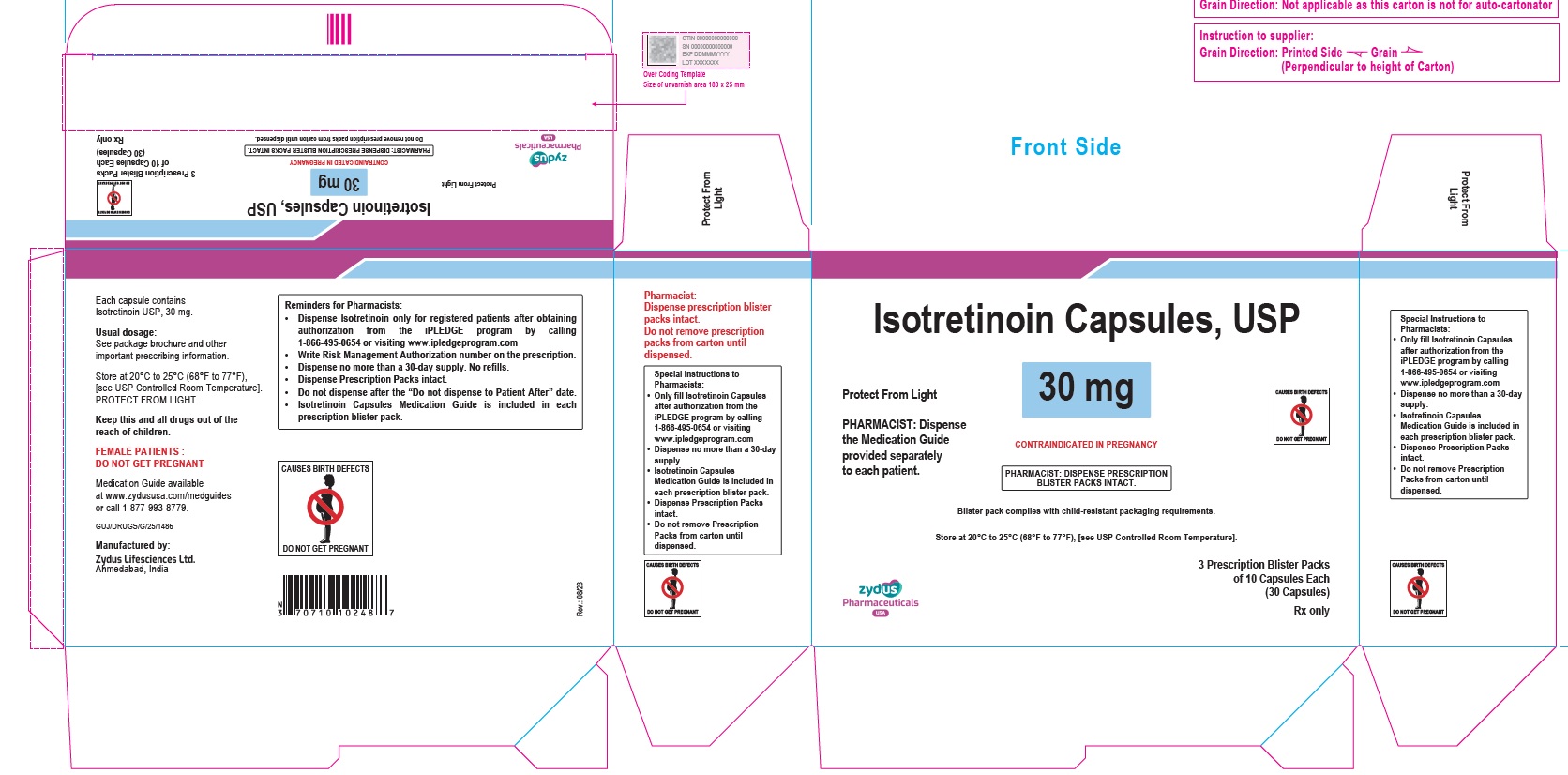
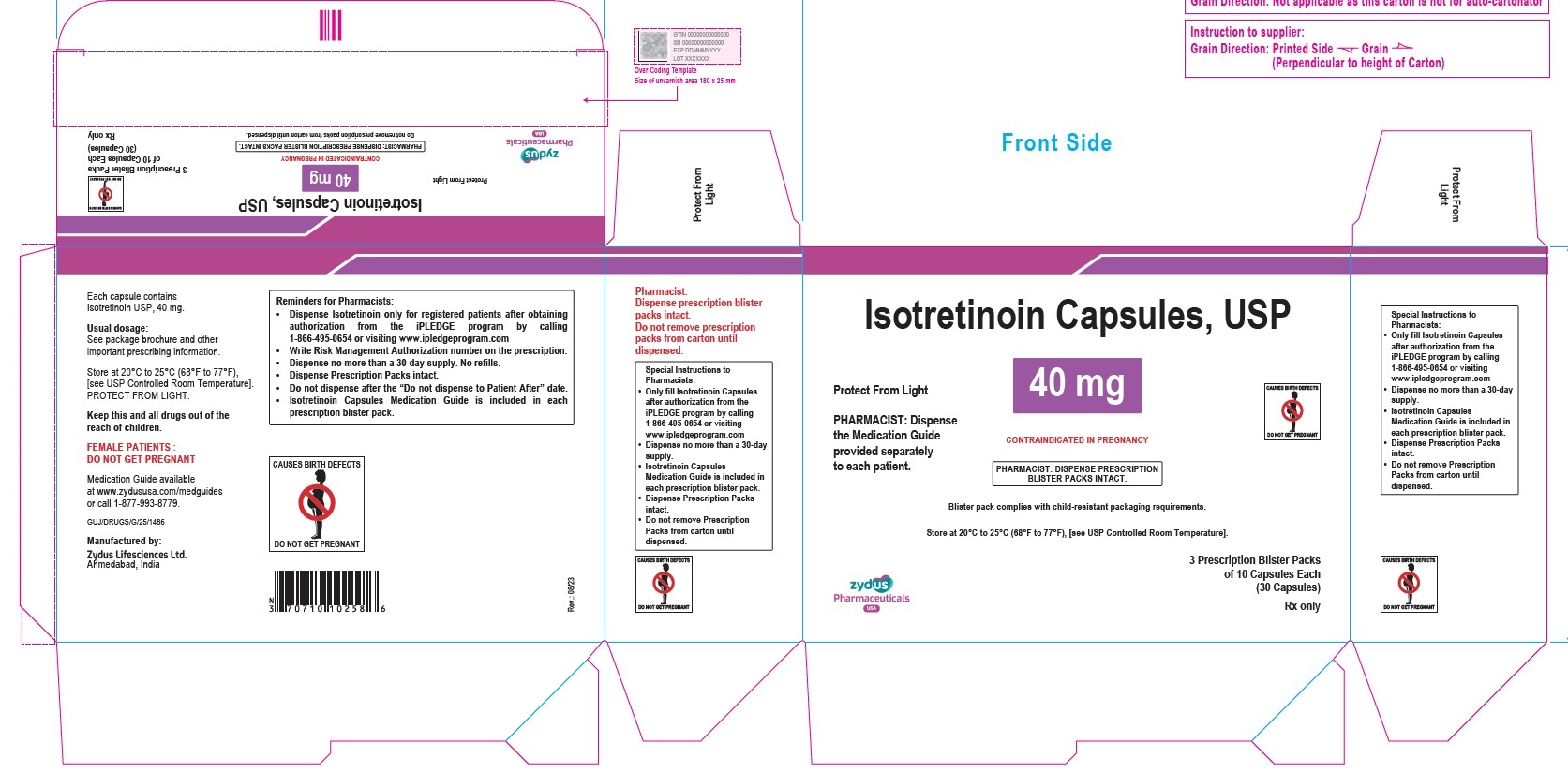
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ISOTRETINOIN
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1557 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 10 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) EDETATE DISODIUM (UNII: 7FLD91C86K) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color BROWN (reddish brown) Score no score Shape OVAL Size 11mm Flavor Imprint Code 1022 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1557-8 3 in 1 CARTON 09/12/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70771-1557-4 10 in 1 CARTON 09/12/2023 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211568 09/12/2023 ISOTRETINOIN
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1558 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 20 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) EDETATE DISODIUM (UNII: 7FLD91C86K) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color YELLOW (CREAM) Score no score Shape OVAL Size 11mm Flavor Imprint Code 1023 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1558-8 3 in 1 CARTON 09/12/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70771-1558-4 10 in 1 CARTON 09/12/2023 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211568 09/12/2023 ISOTRETINOIN
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1559 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 30 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WHITE WAX (UNII: 7G1J5DA97F) AMMONIA (UNII: 5138Q19F1X) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color YELLOW (BEIGE) Score no score Shape OVAL Size 12mm Flavor Imprint Code 1024 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1559-8 3 in 1 CARTON 09/12/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211568 09/12/2023 ISOTRETINOIN
isotretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1560 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOTRETINOIN (UNII: EH28UP18IF) (ISOTRETINOIN - UNII:EH28UP18IF) ISOTRETINOIN 40 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) EDETATE DISODIUM (UNII: 7FLD91C86K) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color BROWN (LIGHT BROWN) Score no score Shape OVAL Size 14mm Flavor Imprint Code 1025 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1560-8 3 in 1 CARTON 09/12/2023 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:70771-1560-4 10 in 1 CARTON 09/12/2023 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211568 09/12/2023 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1557, 70771-1558, 70771-1559, 70771-1560) , MANUFACTURE(70771-1557, 70771-1558, 70771-1559, 70771-1560)