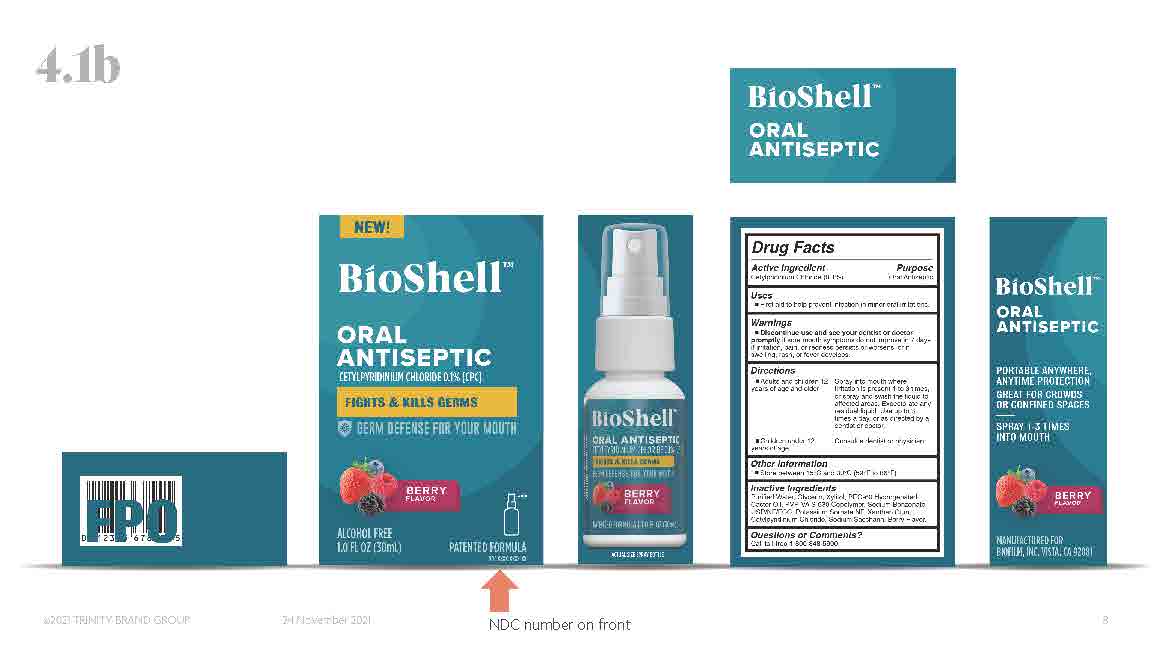
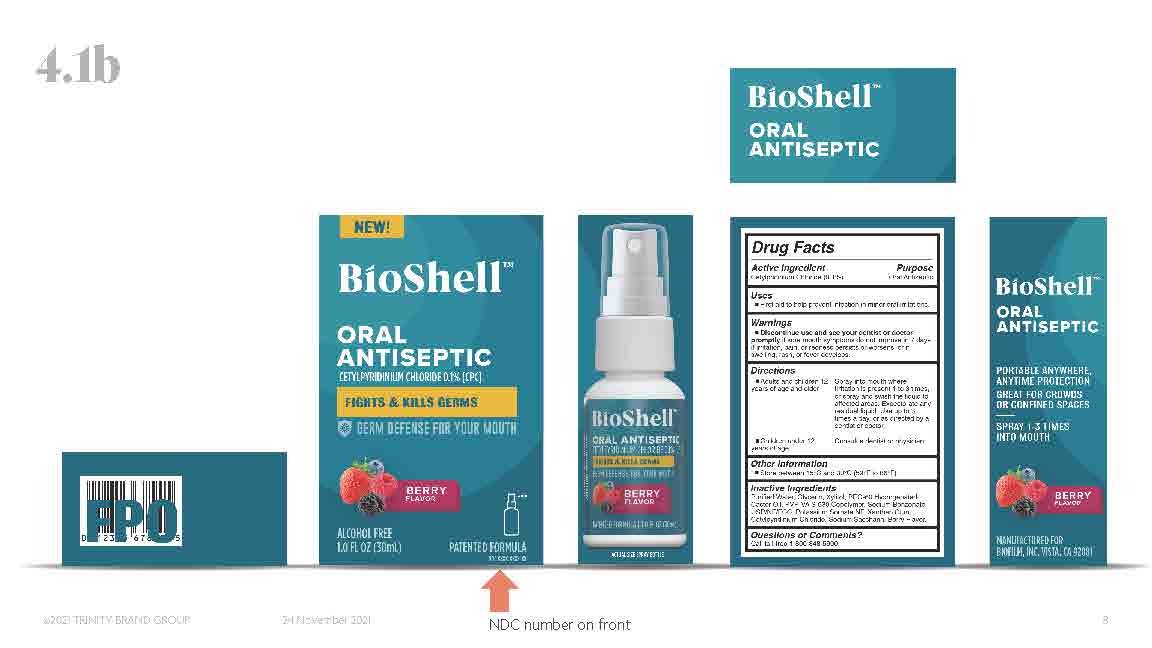
Label: BIOSHELL- cetylpridinium chloride spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 66357-120-01, 66357-120-02 - Packager: BioFilm, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
- Discontinue use and see your dentist or doctor promptly if sore mouth symptoms do not improve in 7 days, if irritation pain, or redness persists or worsens, or if swelling, rash, or fever develops.
- Do not use children under 12 years of age
- Keep out of reach of children
- If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
-
Directions
- Adults and children 12 years of age and older: spray into mouth where irritation is present 1 to 3 times, or spray and swish the liquid to affected areas. Expectorate any residual liquid. Use up to 3 times a day, or as directed by a dentist or doctor.
- Children under 12 years of age: consult a dentist or physician.
- KEEP OUT OF REACH OF CHILDREN
- Other Information
- Inactive ingredients
- Questions or Comments?
- BioShell
-
INGREDIENTS AND APPEARANCE
BIOSHELL
cetylpridinium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66357-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) XYLITOL (UNII: VCQ006KQ1E) PEG-60 HYDROGENATED CASTOR OIL (UNII: 02NG325BQG) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66357-120-02 1 in 1 BOX 05/02/2022 1 NDC:66357-120-01 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/02/2022 Labeler - BioFilm, Inc. (790780258) Establishment Name Address ID/FEI Business Operations BioFilm, Inc. 790780258 manufacture(66357-120)