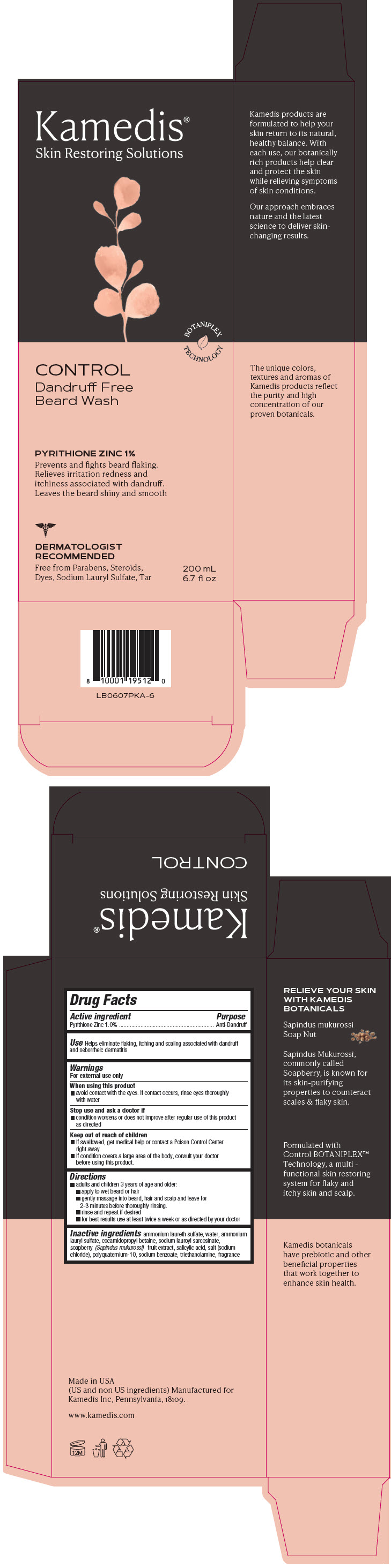
Label: CONTROL DANDRUFF- FREE BEARD WASH- pyrithione zinc shampoo
- NDC Code(s): 50718-0035-1
- Packager: Kamedis Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL - 200 mL Bottle Carton
Kamedis®
Skin Restoring SolutionsBOTANIPLEX
TECHNOLOGYCONTROL
Dandruff Free
Beard WashPYRITHIONE ZINC 1%
Prevents and fights beard flaking.
Relieves irritation redness and
itchiness associated with dandruff.
Leaves the beard shiny and smoothDERMATOLOGIST
RECOMMENDED
Free from Parabens, Steroids,
Dyes, Sodium Lauryl Sulfate, Tar200 mL
6.7 fl oz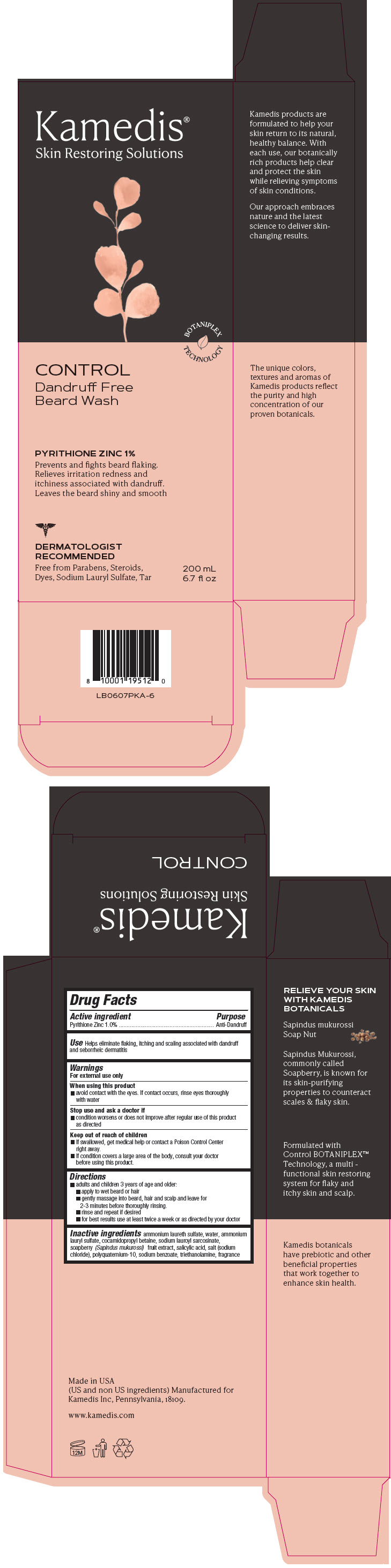
-
INGREDIENTS AND APPEARANCE
CONTROL DANDRUFF- FREE BEARD WASH
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50718-0035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SALICYLIC ACID (UNII: O414PZ4LPZ) SODIUM CHLORIDE (UNII: 451W47IQ8X) SAPINDUS MUKOROSSI FRUIT (UNII: 66H9NW427Y) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) WATER (UNII: 059QF0KO0R) AMMONIUM LAURETH-2 SULFATE (UNII: 698O4Z48G6) POLYQUATERNIUM-10 (10000 MPA.S AT 2%) (UNII: PI1STR9QYH) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50718-0035-1 1 in 1 CARTON 12/01/2022 1 200 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M032 12/01/2022 Labeler - Kamedis Inc (080311300) Establishment Name Address ID/FEI Business Operations Biogenesis Inc 069117328 MANUFACTURE(50718-0035) Establishment Name Address ID/FEI Business Operations Fragrance Manufacturing Inc 793406000 MANUFACTURE(50718-0035)