Label: MED NAP CLEANSING TOWELETTE- benzalkonium chloride liquid
- NDC Code(s): 0924-0243-00, 0924-0243-01
- Packager: Acme United Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredient
- Purpose
- Use:
- Caution:
- Warnings
- Stop use
- ASK DOCTOR
- Directions
- Other Information
- Inactive Ingredients
- QUESTIONS
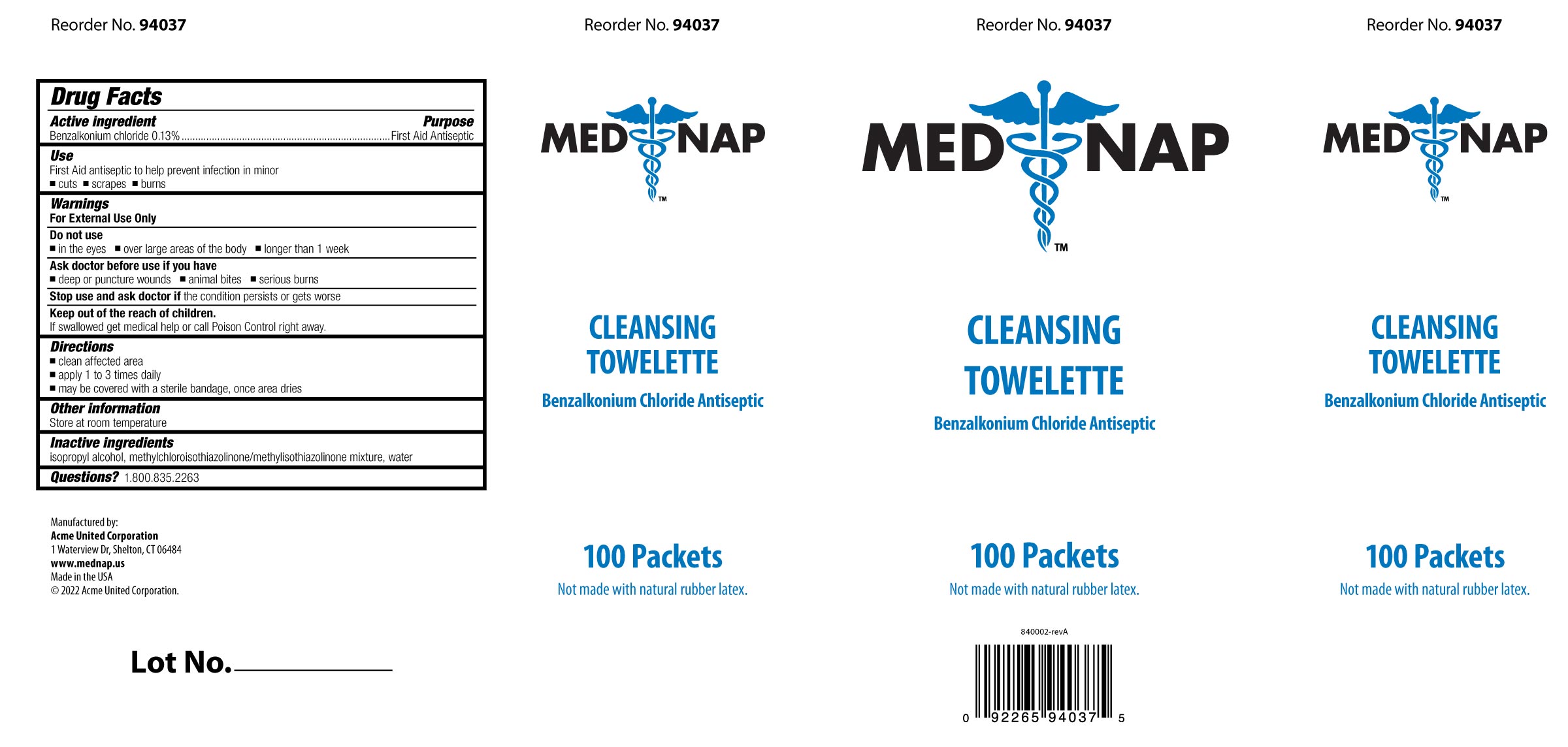
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MED NAP CLEANSING TOWELETTE
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-0243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.0018 mg in 1.35 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-0243-00 1.35 mL in 1 PACKET; Type 0: Not a Combination Product 03/02/2022 2 NDC:0924-0243-01 100 in 1 BOX 03/02/2022 2 1.35 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 03/02/2022 Labeler - Acme United Corporation (001180207) Establishment Name Address ID/FEI Business Operations Acme United Corporation 117825595 manufacture(0924-0243)

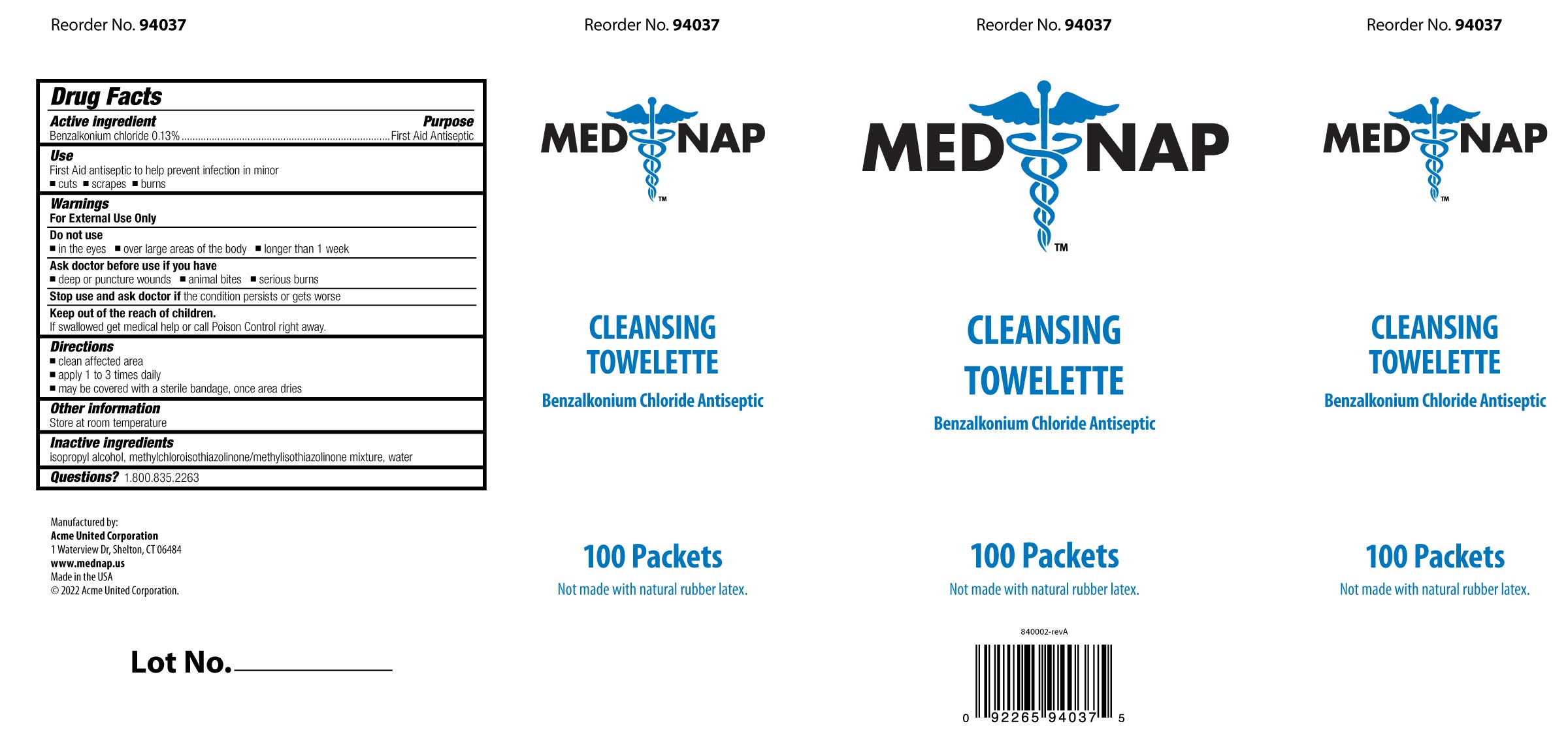
 Box Image
Box Image