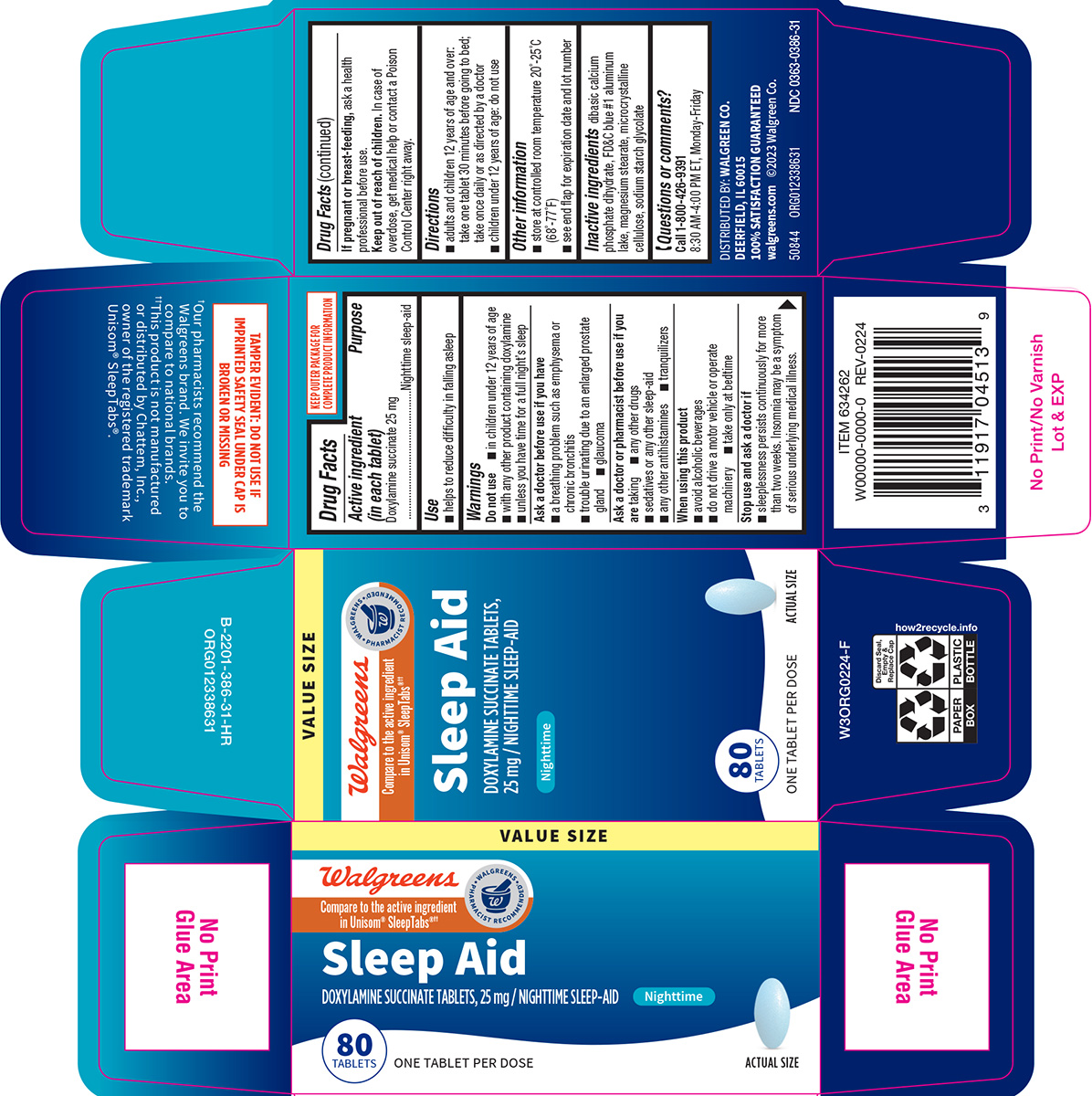
Label: SLEEP AID- doxylamine succinate tablet
- NDC Code(s): 0363-0386-31
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Do not use
- in children under 12 years of age
- with any other product containing doxylamine
- unless you have time for a full night’s sleep
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking
- sedatives or any other sleep-aid
- tranquilizers
- any other antihistamines
- any other drugs
When using this product
- avoid alcoholic beverages
- do not drive a motor vehicle or operate machinery
- take only at bedtime
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Walgreens
Compare to the active ingredient
in Unisom® SleepTabs®††Sleep Aid
DOXYLAMINE SUCCINATE TABLETS,
25 mg / NIGHTTIME SLEEP-AID
Nighttime80
TABLETSONE TABLET PER DOSE ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP IS
BROKEN OR MISSING†Our pharmacists recommend the
Walgreens brand. We invite you to
compare to national brands.
††This product is not manufactured
or distributed by Chattem, Inc.,
owner of the registered trademark
Unisom® SleepTabs®.DISTRIBUTED BY: WALGREEN CO.
DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
walgreens.com ©2023 Walgreen Co.50844 ORG012338631 NDC 0363-0386-31
WALGREENS 44-386
-
INGREDIENTS AND APPEARANCE
SLEEP AID
doxylamine succinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0386 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 25 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color blue Score no score Shape OVAL Size 10mm Flavor Imprint Code 44;386 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0386-31 1 in 1 CARTON 04/05/2024 1 80 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040564 04/05/2024 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0363-0386) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0363-0386) , pack(0363-0386) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0363-0386) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0363-0386)