Label: TRIAMULOX- tiamulin hydrogen fumarate liquid
- NDC Code(s): 54771-8729-0, 54771-8729-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
Drug Label Information
Updated October 12, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)
-
Description
Triamulox (tiamulin hydrogen fumarate) Liquid Concentrate is a solution containing 12.3% tiamulin hydrogen fumarate (w/v) in an aqueous solution. The active ingredient, tiamulin, chemically is 14-desoxy-14-[(2-diethylaminoethyl) mercaptoacetoxy] mutilin hydrogen fumarate, a semi-synthetic diterpene antibiotic. Triamulox is for use only in preparing medicated drinking water for swine.
- Actions
-
Indications
Triamulox, when administered in the drinking water for ve consecutive days, is an effective antibiotic for the treatment of swine dysentery associated with Brachyspira (formerly Serpulina or Treponema) hyodysenteriae susceptible to tiamulin at a dose level of 3.5 mg tiamulin hydrogen fumarate per pound of body weight daily and for treatment of swine pneumonia due to Actinobacillus pleuropneumoniae susceptible to tiamulin when given at 10.5 mg tiamulin hydrogen fumarate per pound of body weight daily.
Contraindications: Swine being treated with Triamulox should not have access to feeds containing polyether ionophores (e.g., monensin, lasalocid, narasin, salinomycin and semduramicin) as adverse reactions may occur.
-
Warnings
Keep out of reach of children. Avoid contact with skin. Direct contact with skin or mucous membranes may cause irritation.
Residue Warning: Withdraw medicated water 3 days before slaughter after treatment at 3.5 mg per pound and 7 days before slaughter following treatment at 10.5 mg per pound body weight.
Caution: For use in drinking water of swine only - Not for use in humans. Prepare fresh medicated water daily. Use as the only source of drinking water for 5 days. The effects of tiamulin on swine reproductive performance, pregnancy and lactation have not been determined.
Adverse Reactions: Overdoses of Triamulox have sometimes produced transitory salivation, vomiting and an apparent calming effect on the pig. If signs of toxicity occur, discontinue use of medicated water and replace with clean, fresh water.
In rare cases, redness of the skin primarily over the ham and underline has been observed during medication. If these signs appear, discontinue use of this drug. Provide ample clean drinking water. Thoroughly rinse (hose down) the housing to remove urine and feces from animal contact surfaces or move the animals to clean pens. If the condition persists, consult your veterinarian.
Studies to evaluate the safety of the water soluble form of tiamulin in breeding swine have not been done.
-
Use Directions
The concentration of tiamulin in the drinking water must be adjusted to compensate for variation in water consumption due to weight or size of the pig, environmental temperature and other factors. It is important that pigs receive the proper drug dose, 3.5 mg tiamulin hydrogen fumarate per pound for swine dysentery or 10.5 mg tiamulin hydrogen fumarate per pound for swine pneumonia, each day for 5 consecutive days.

Directions for preparing Triamulox Liquid Concentrate medicated solutions: Determine the amount of Triamulox needed to medicate the desired volume of drinking water at the proper concentration. Carefully measure out this amount, add it to the water and stir to thoroughly mix.
Table 2 Triamulox Liquid Concentrate
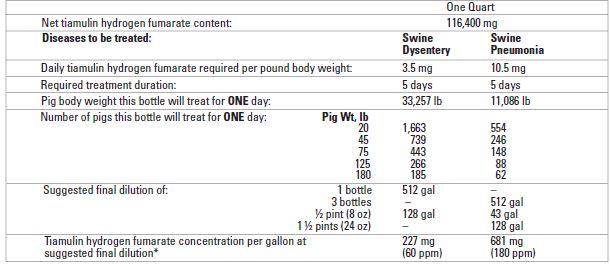
*Note: Increase or decrease dilution rate as required to obtain proper daily drug dose.
Directions for using Triamulox Liquid Concentrate in medicated proportioners: One quart of Triamulox mixed with water to make four gallons of stock solution and this stock solution metered at one fluid ounce per gallon will provide 227 mg of tiamulin hydrogen fumarate per gallon to 512 gallons of drinking water for treatment of swine dysentery. Three quarts of Triamulox mixed with water to make four gallons of stock solution and this stock solution metered at one -fluid ounce per gallon will provide 681 mg tiamulin hydrogen fumarate per gallon to a total of 512 gallons of drinking water for treatment of swine pneumonia. One pint of Triamulox mixed with water to make two gallons of stock solution and this stock solution metered at one fluid ounce per gallon will provide 227 mg of tiamulin hydrogen fumarate per gallon to 256 gallons of drinking water for treatment of swine dysentery. Use three pints of Triamulox in two gallons of stock solution to be metered at one fluid ounce per gallon to deliver 681 mg per gallon to a total of 256 gallons of drinking water for treatment of swine pneumonia. One-half pint (8 -fluid ounces) of Triamulox diluted with water to make one gallon of stock solution and this stock solution metered at one fluid ounce of drinking water with a medication proportioner will provide 227 mg of tiamulin hydrogen fumarate per gallon to 128 gallons of drinking water for treatment of swine dysentery. Use one and one-half pints of Triamulox per gallon of stock solution to be metered at one fluid ounce per gallon to provide 681 mg per gallon to 128 gallons of drinking water for treatment of swine pneumonia.
In barrels or tanks: Three fluid ounces of Triamulox will medicate 48 gallons of drinking water at 227 mg per gallon for treatment of swine dysentery or 16 gallons at 681 mg per gallon for treatment of swine pneumonia. Measure Triamulox carefully, pour into the proper amount of water and thoroughly mix. The concentration of tiamulin hydrogen fumarate in the stock solution and in the drinking water delivered must be adjusted to compensate for variation in water consumption by pigs due to body weight, environmental and other factors. It is important that the pigs receive the proper drug dose of 3.5 mg of tiamulin hydrogen fumarate per pound of body weight daily for 5 consecutive days for treatment of swine dysentery or a dose of 10.5 mg per pound body weight daily for 5 consecutive days for treatment of swine pneumonia.
Attention: If no response to treatment is obtained within 5 days re-establish the diagnosis. Failure of response may be related to the presence of non-susceptible organisms of other complicating disease conditions. Because of the tendency for the disease to recur on premises with a history of swine dysentery or with swine pneumonia, a control program should be implemented after treatment. Drugs are not substitutes for proper sanitary measures or good management, but should be used in conjunction with such practices.
- How Supplied
- Storage
- other information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TRIAMULOX
tiamulin hydrogen fumarate liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-8729 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIAMULIN FUMARATE (UNII: ION1Q02ZCX) (TIAMULIN - UNII:E38WZ4U54R) TIAMULIN FUMARATE 116.4 g in 946 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8729-0 946 mL in 1 BOTTLE 2 NDC:54771-8729-1 4730 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200512 10/10/2016 Labeler - Zoetis Inc. (828851555)


