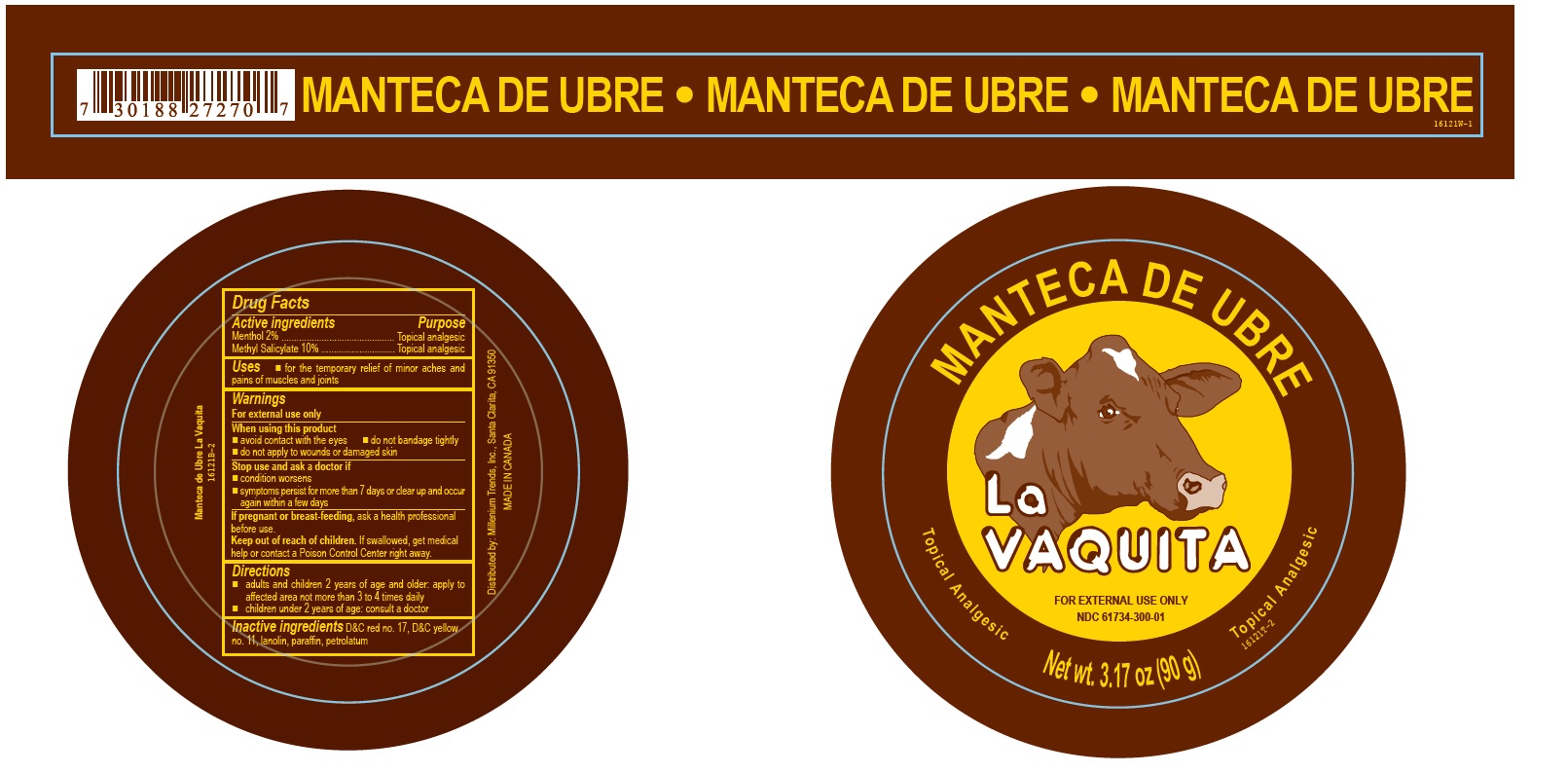
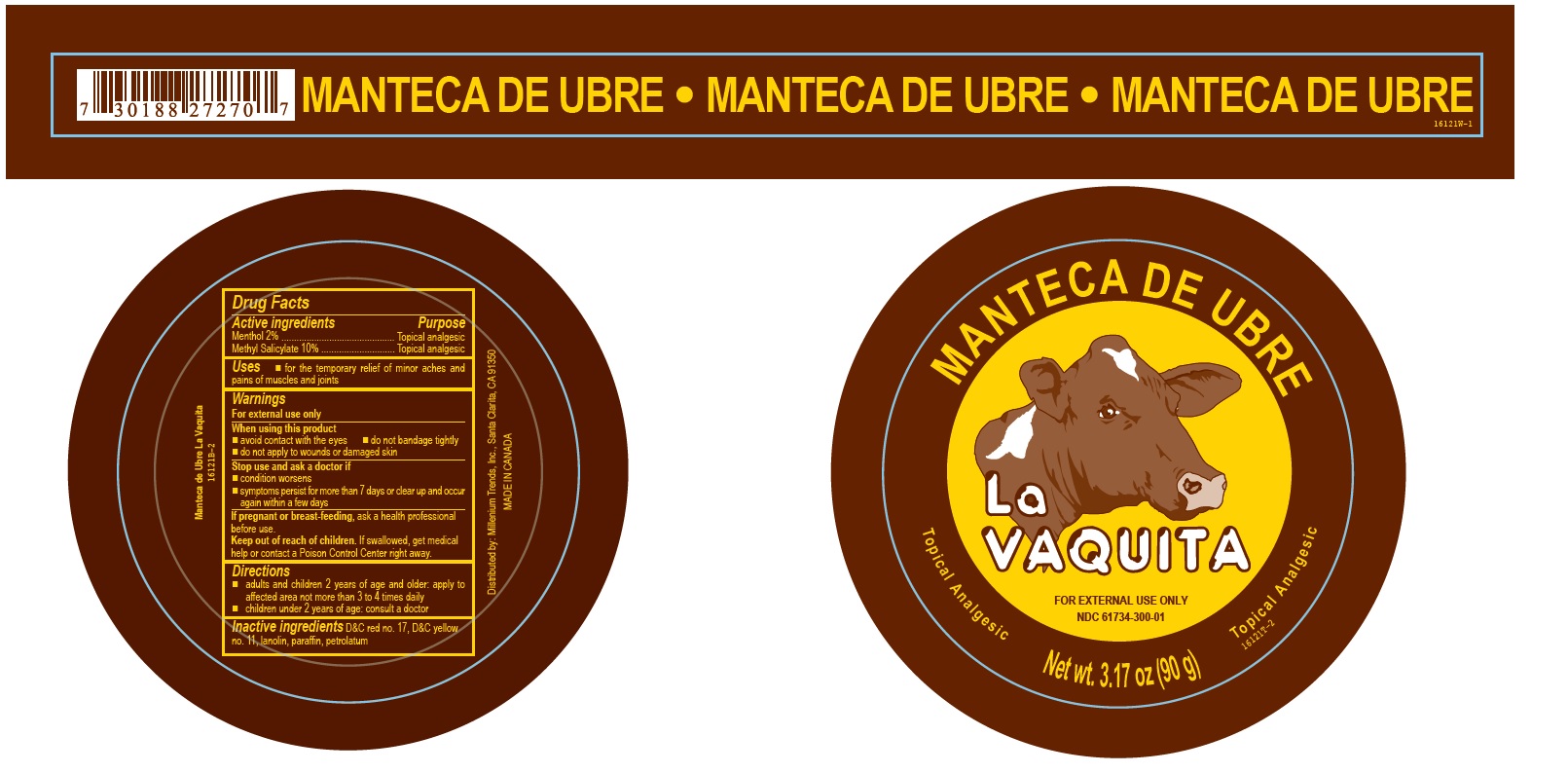
Label: LA VAQUITA NEW REGULAR- methyl salicylate ointment
- NDC Code(s): 61734-300-01, 61734-300-02
- Packager: Delon Laboratories (1990) Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA VAQUITA NEW REGULAR
methyl salicylate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61734-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) LANOLIN (UNII: 7EV65EAW6H) D&C RED NO. 17 (UNII: ND733RX3JN) D&C YELLOW NO. 11 (UNII: 44F3HYL954) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61734-300-01 90 g in 1 CAN; Type 0: Not a Combination Product 03/01/2011 2 NDC:61734-300-02 100 g in 1 JAR; Type 0: Not a Combination Product 07/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/01/2011 Labeler - Delon Laboratories (1990) Ltd (248364184) Establishment Name Address ID/FEI Business Operations Laboratoires Delon 208896216 label(61734-300) , manufacture(61734-300) , pack(61734-300)