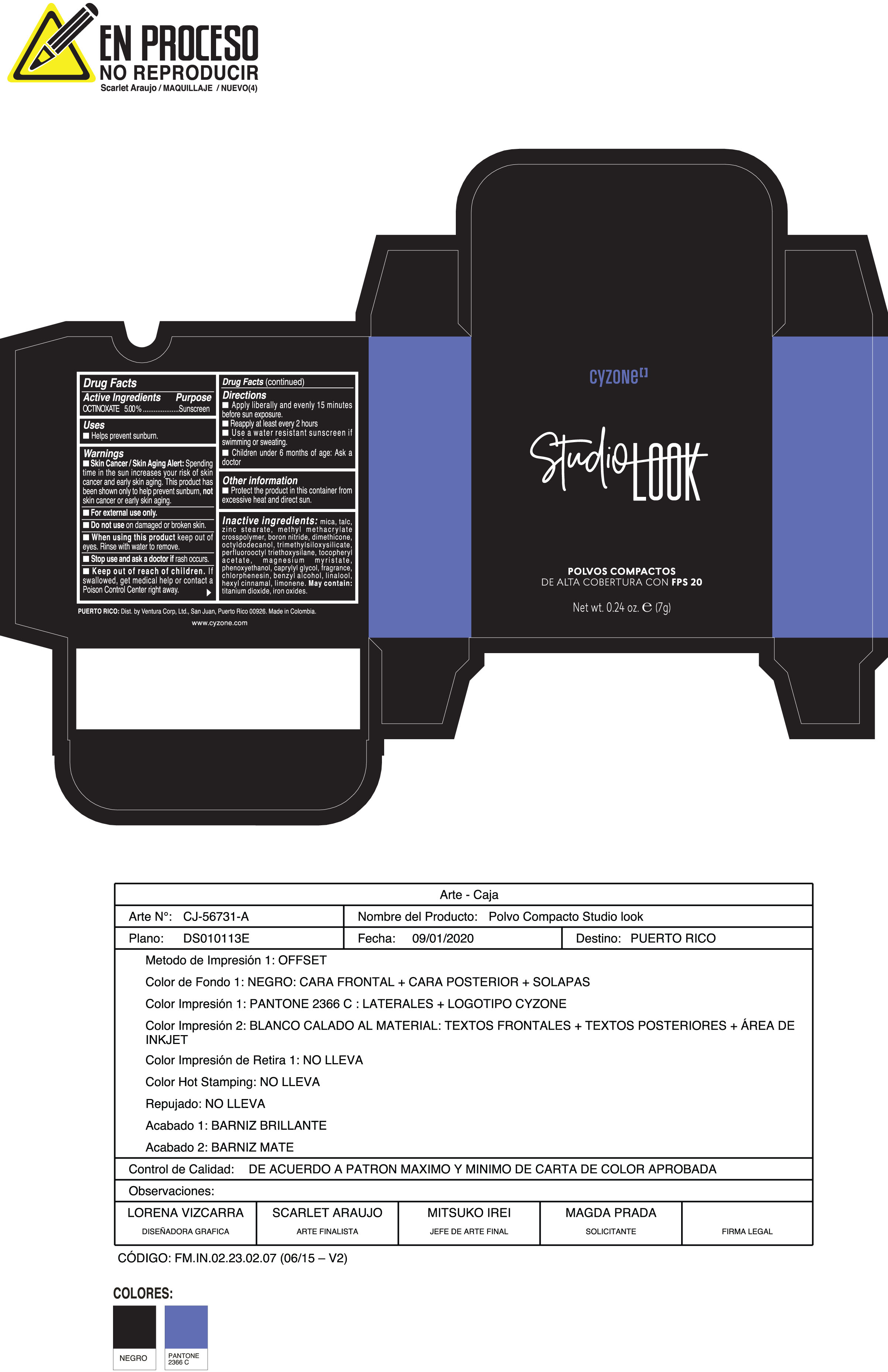
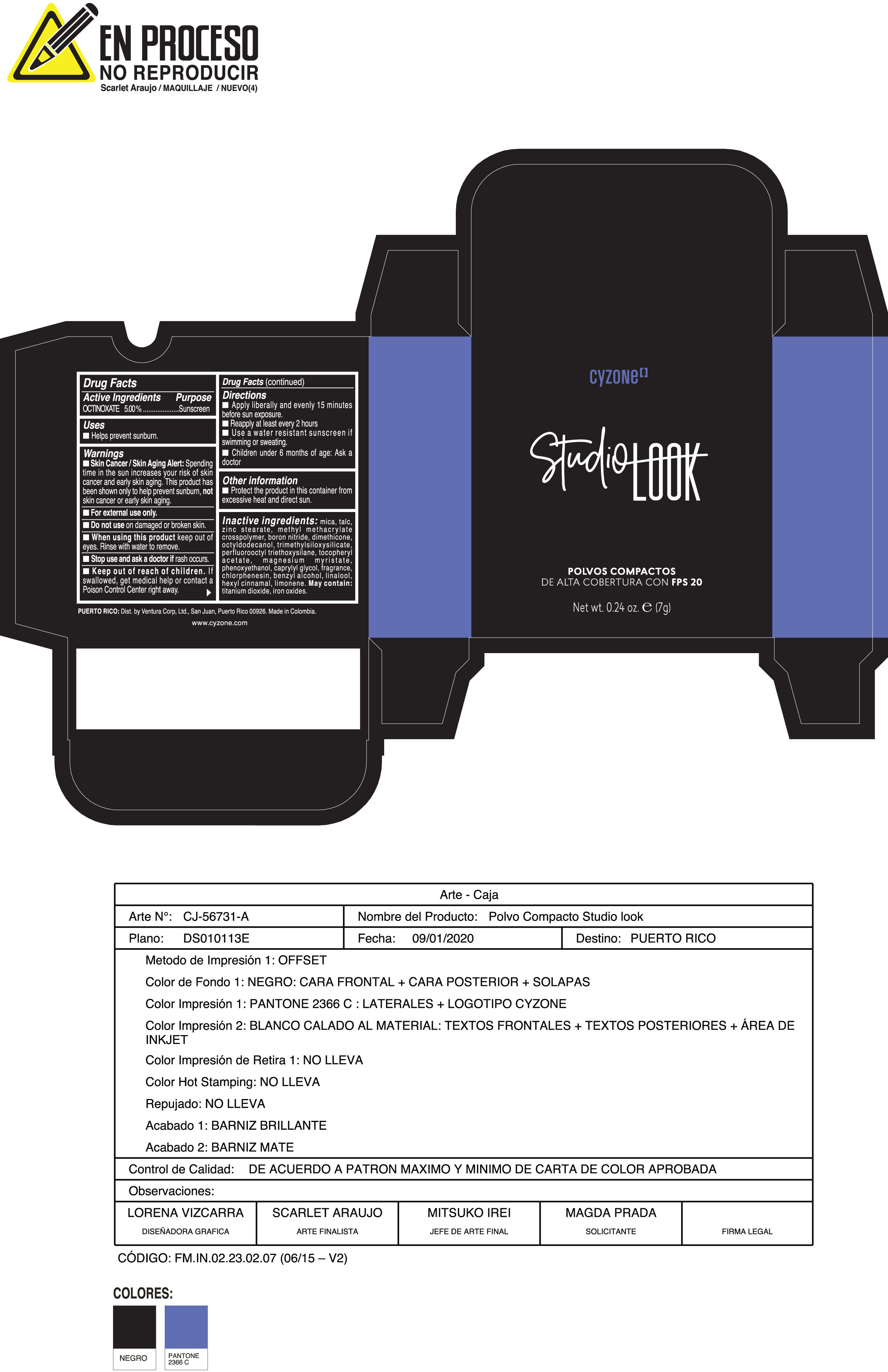
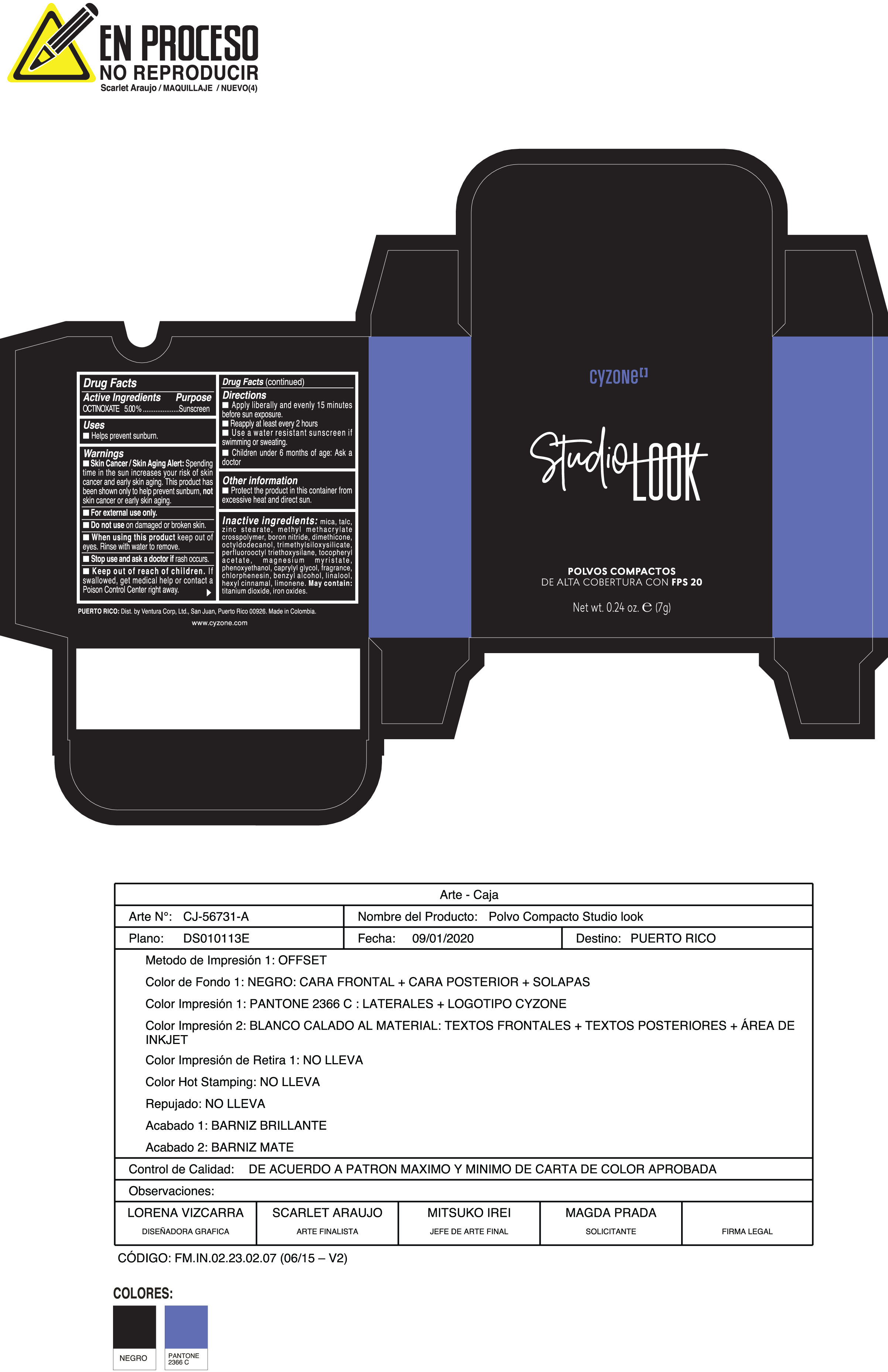
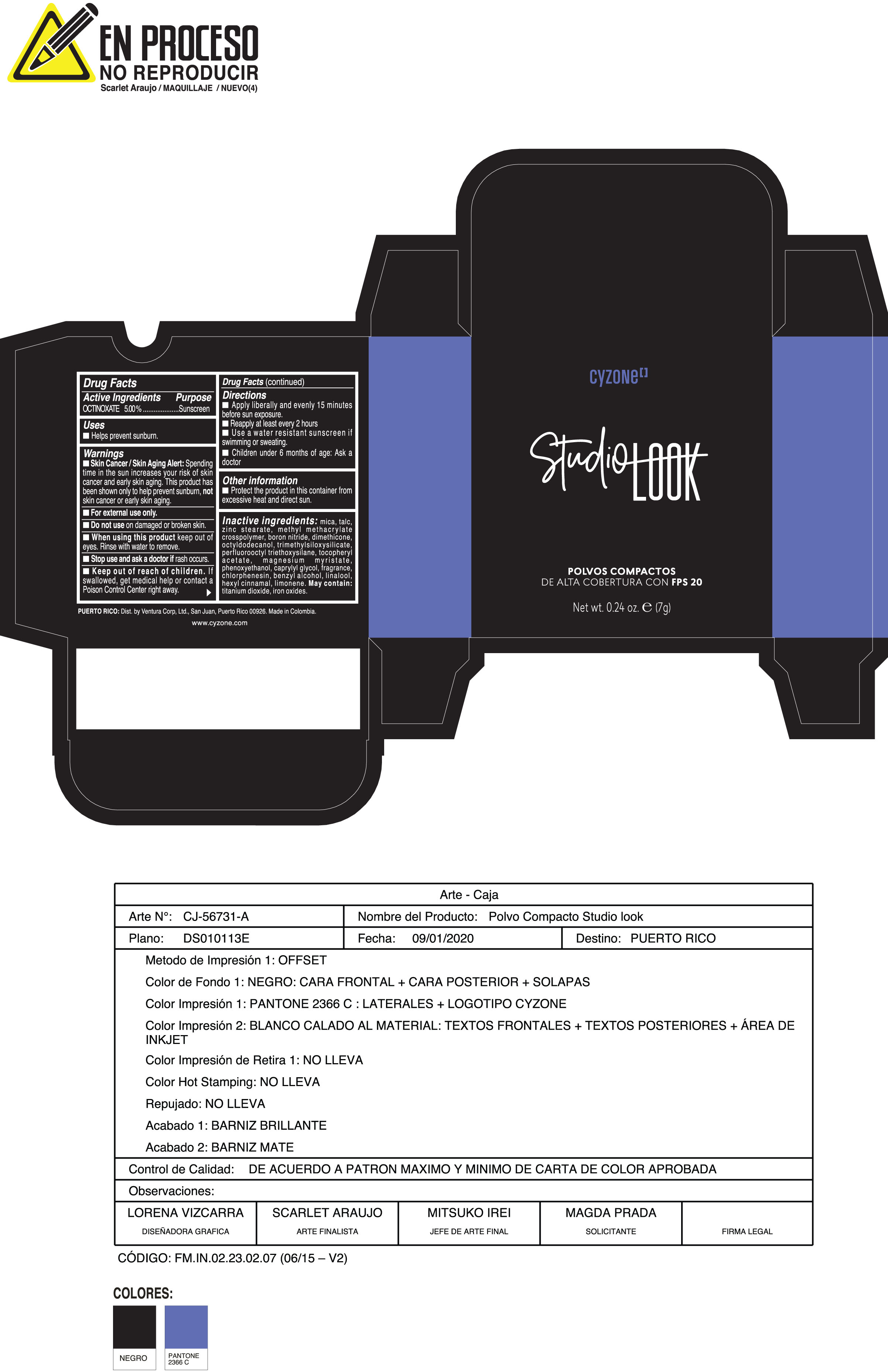
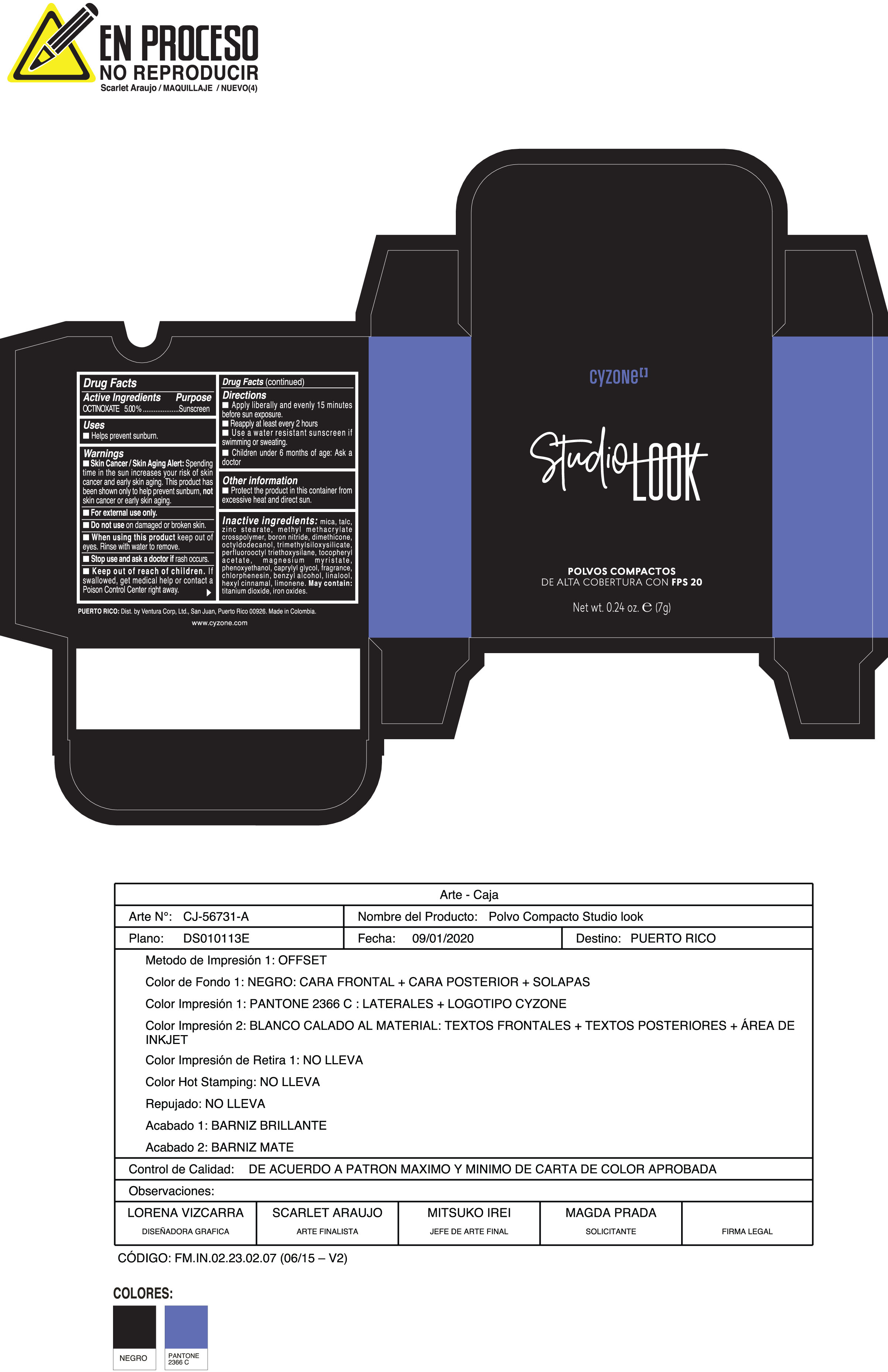
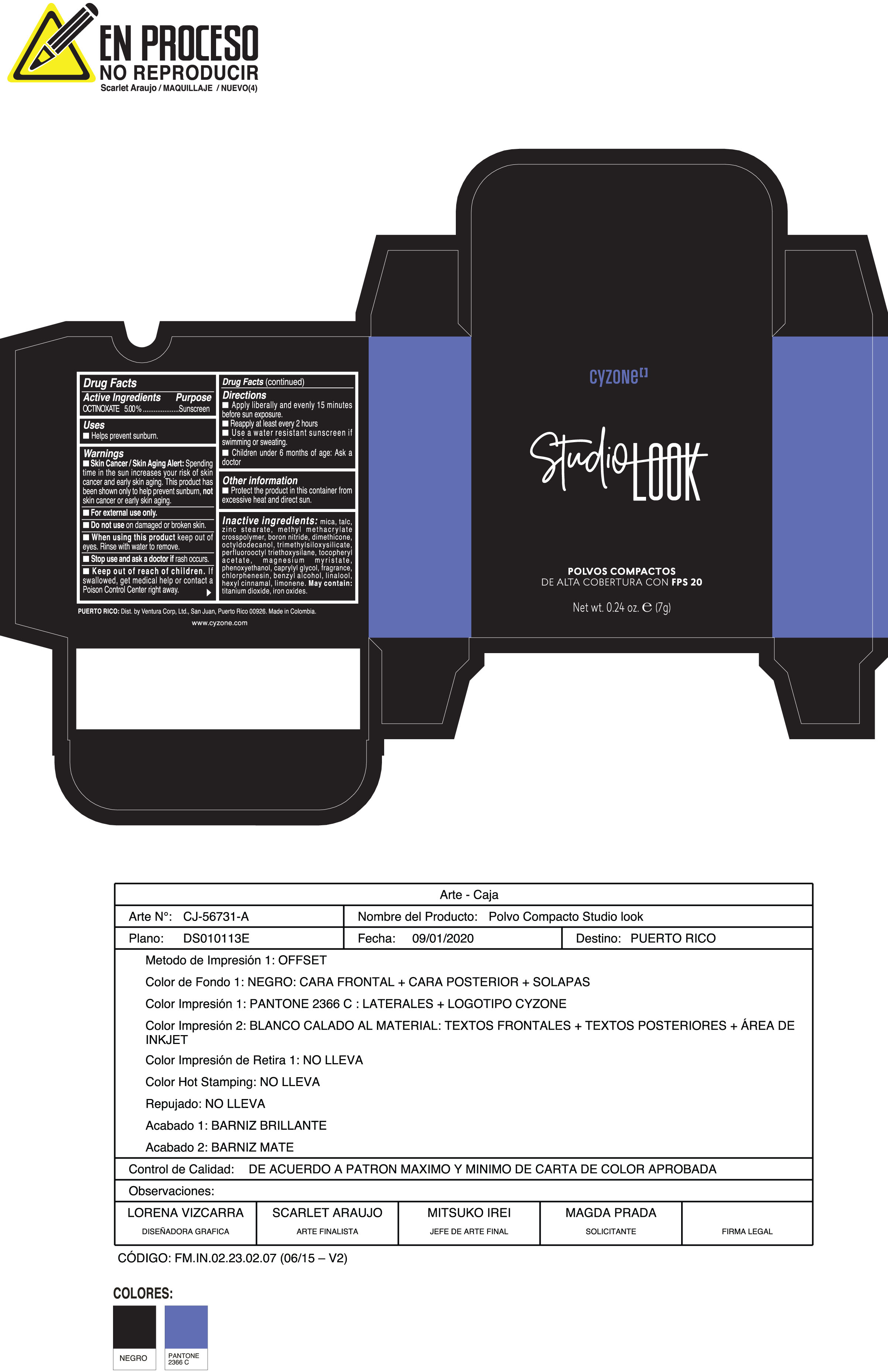
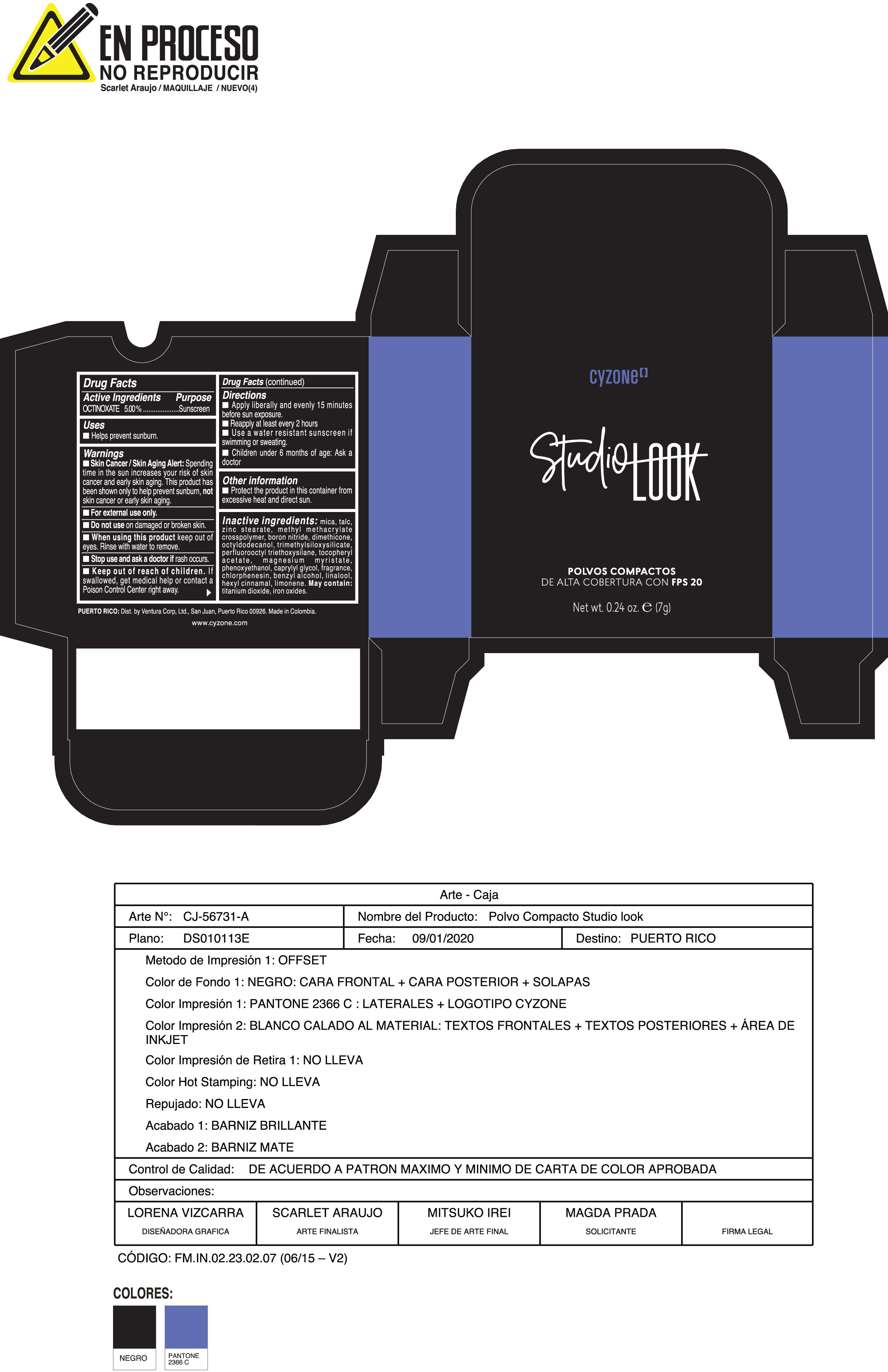
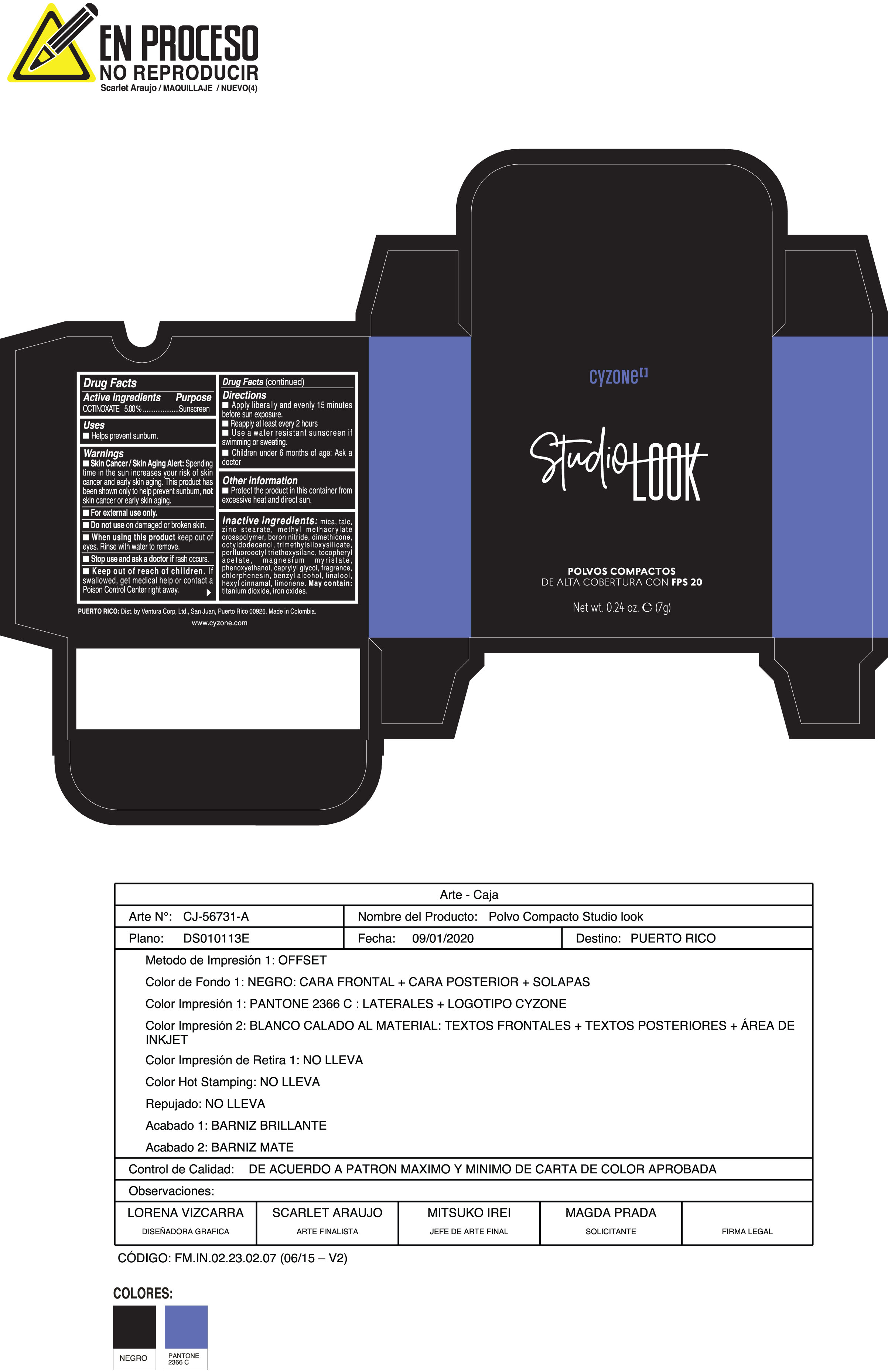
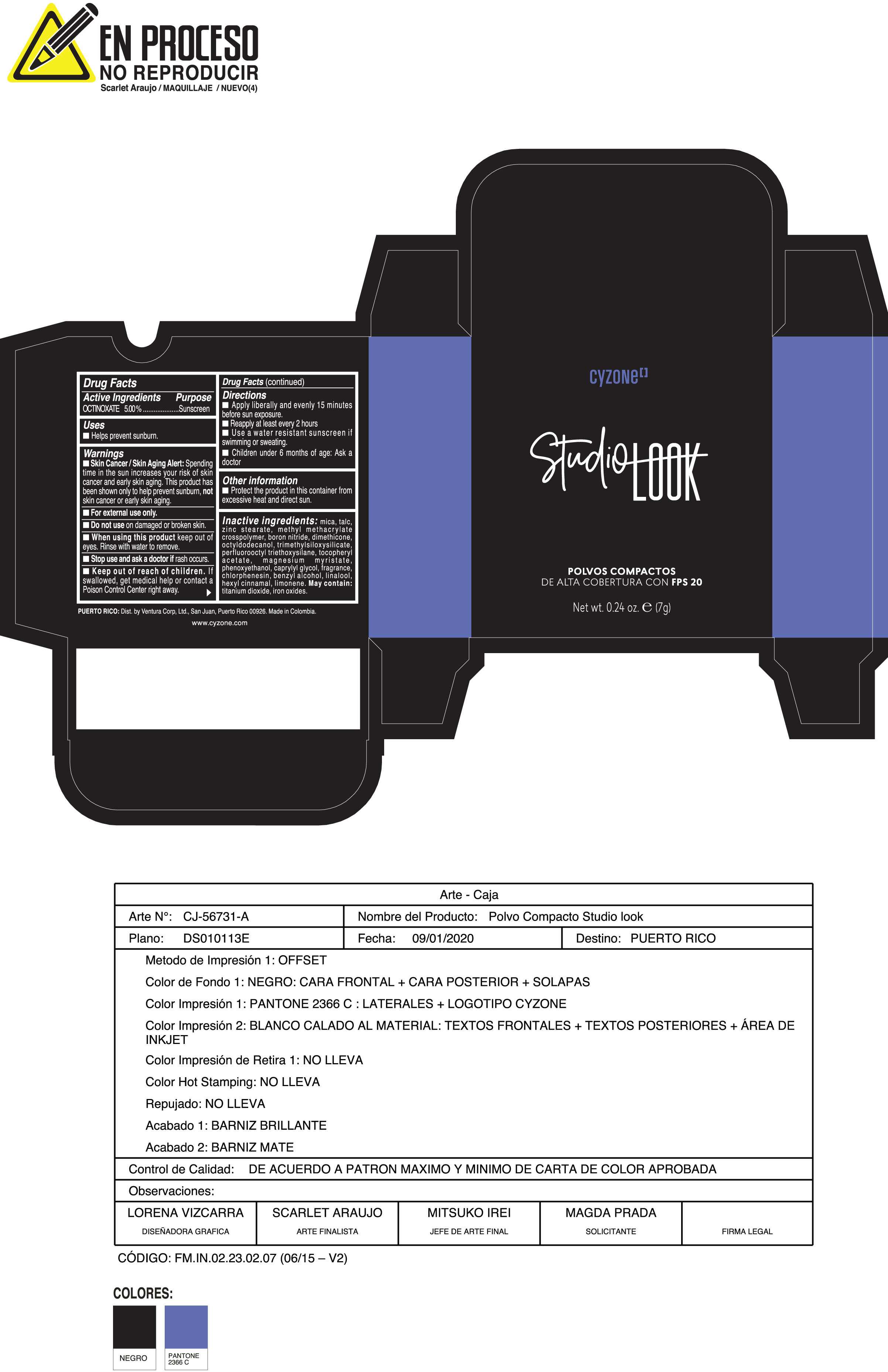
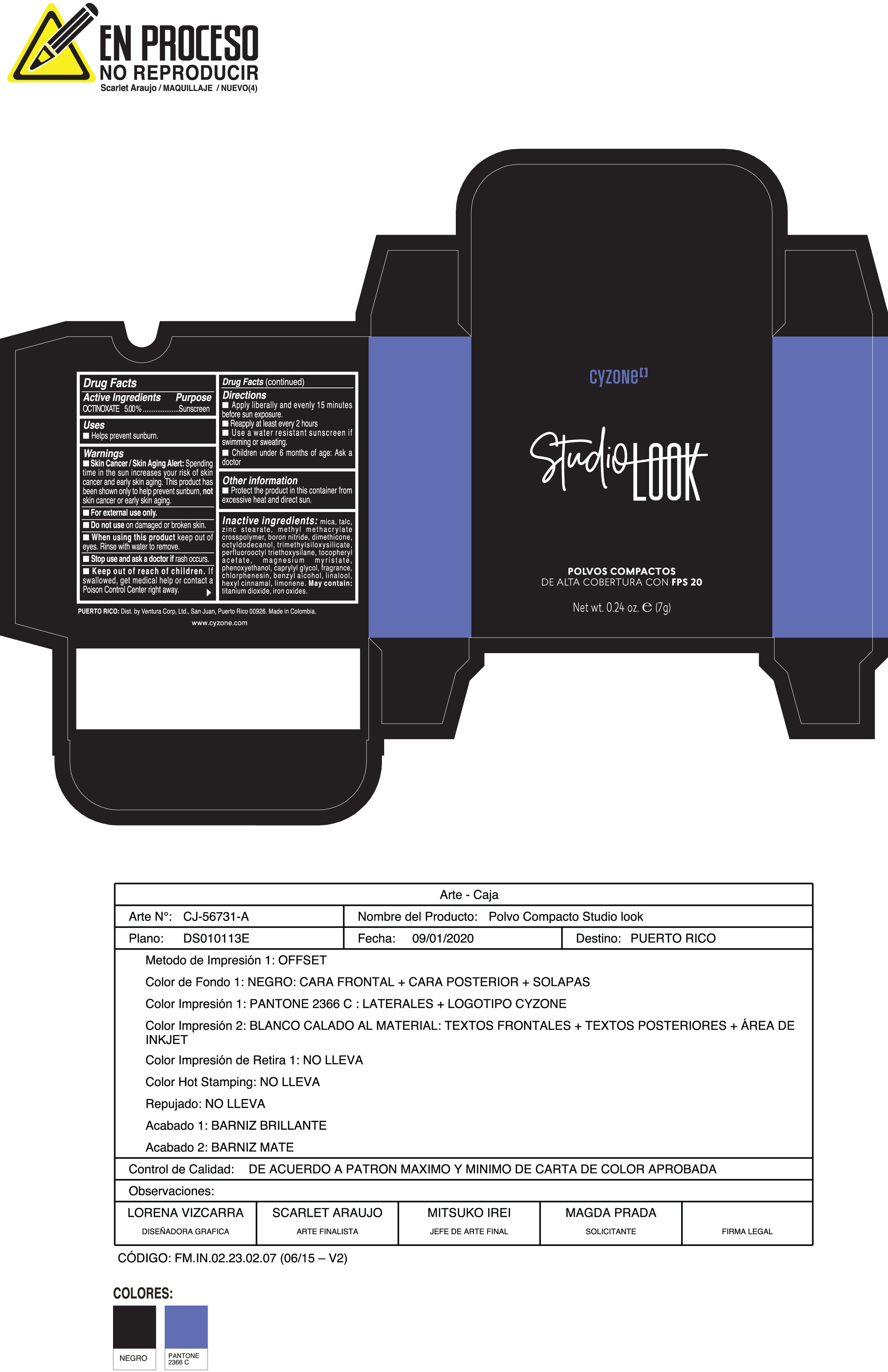
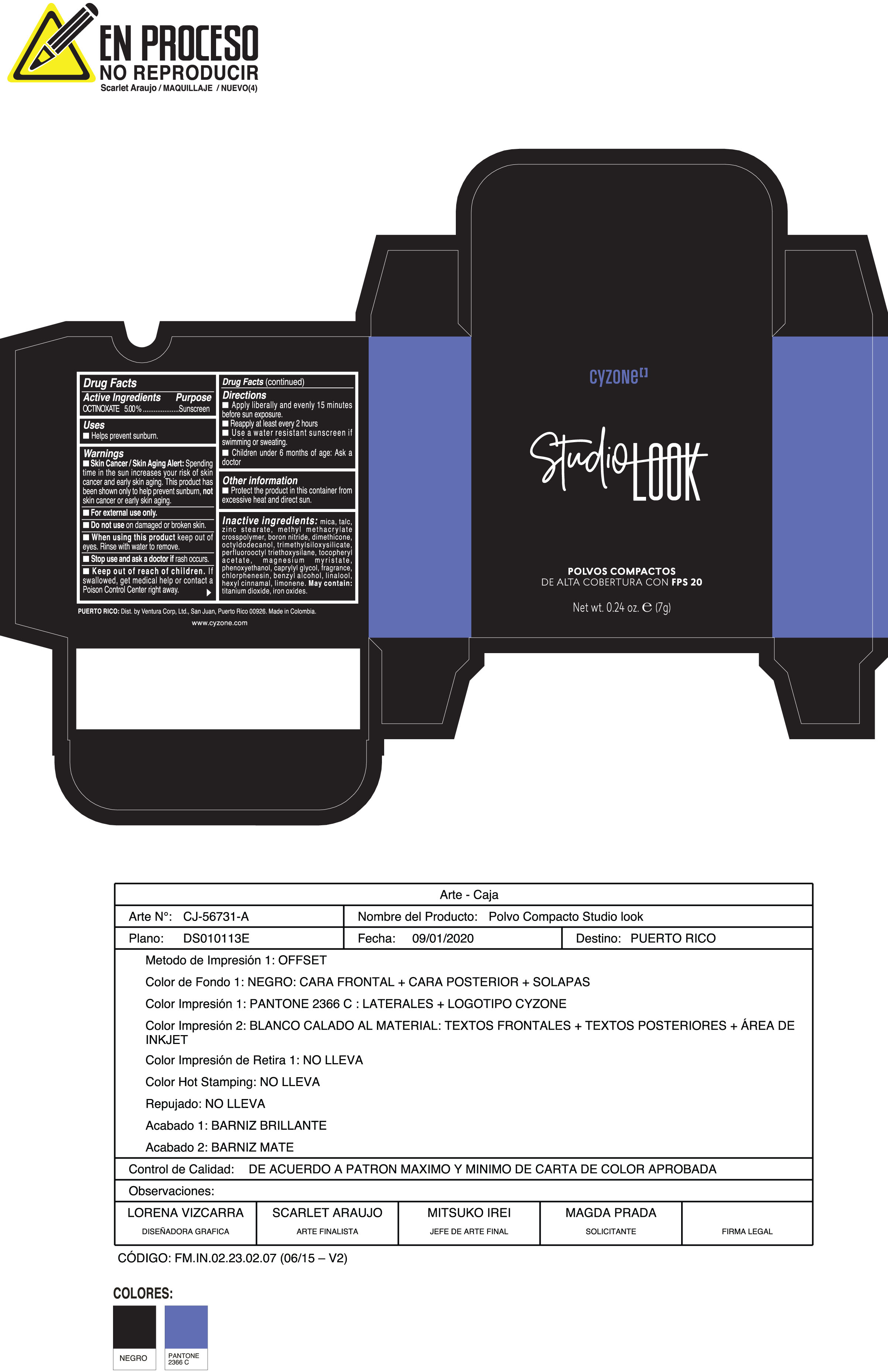
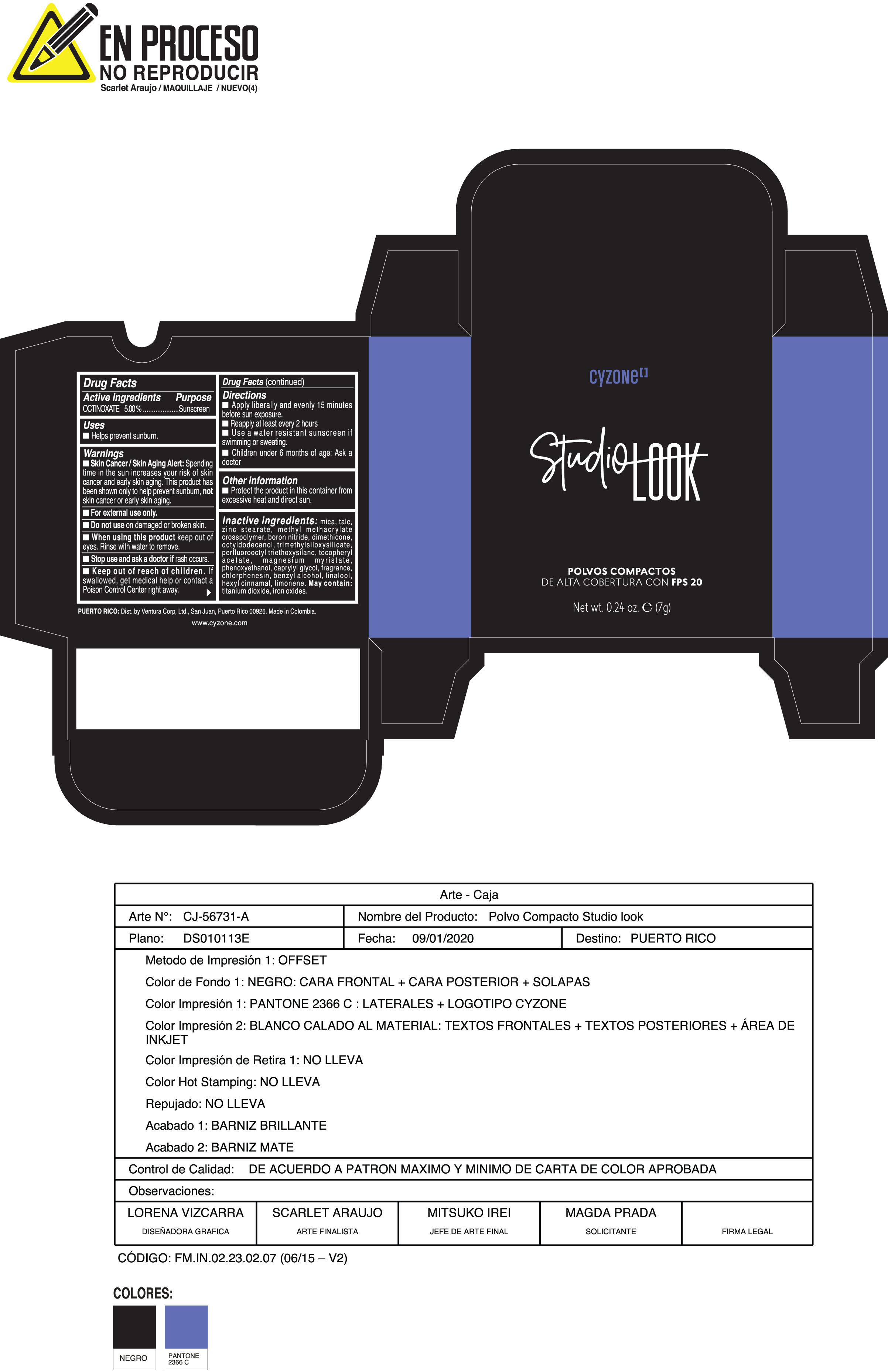
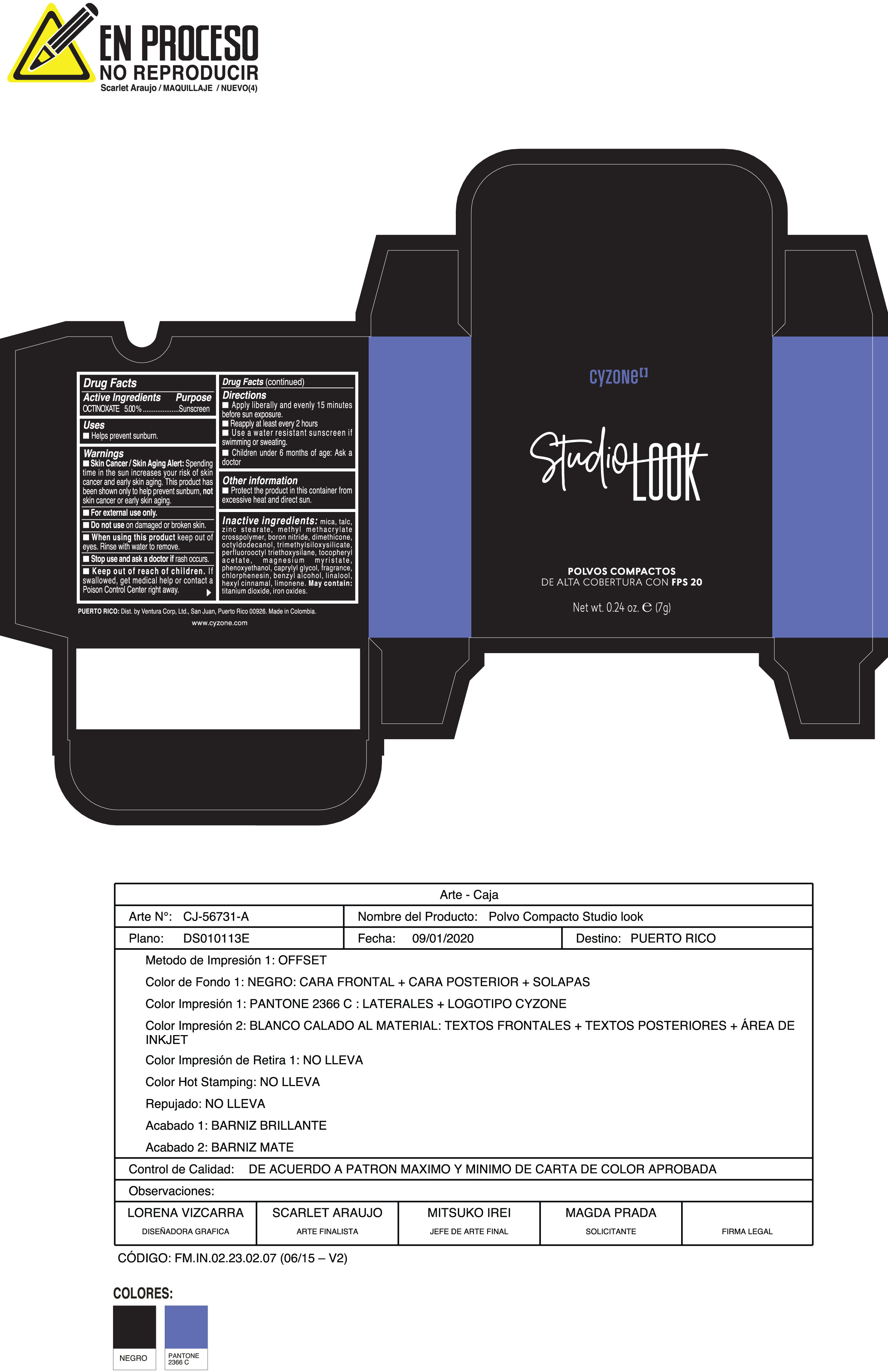
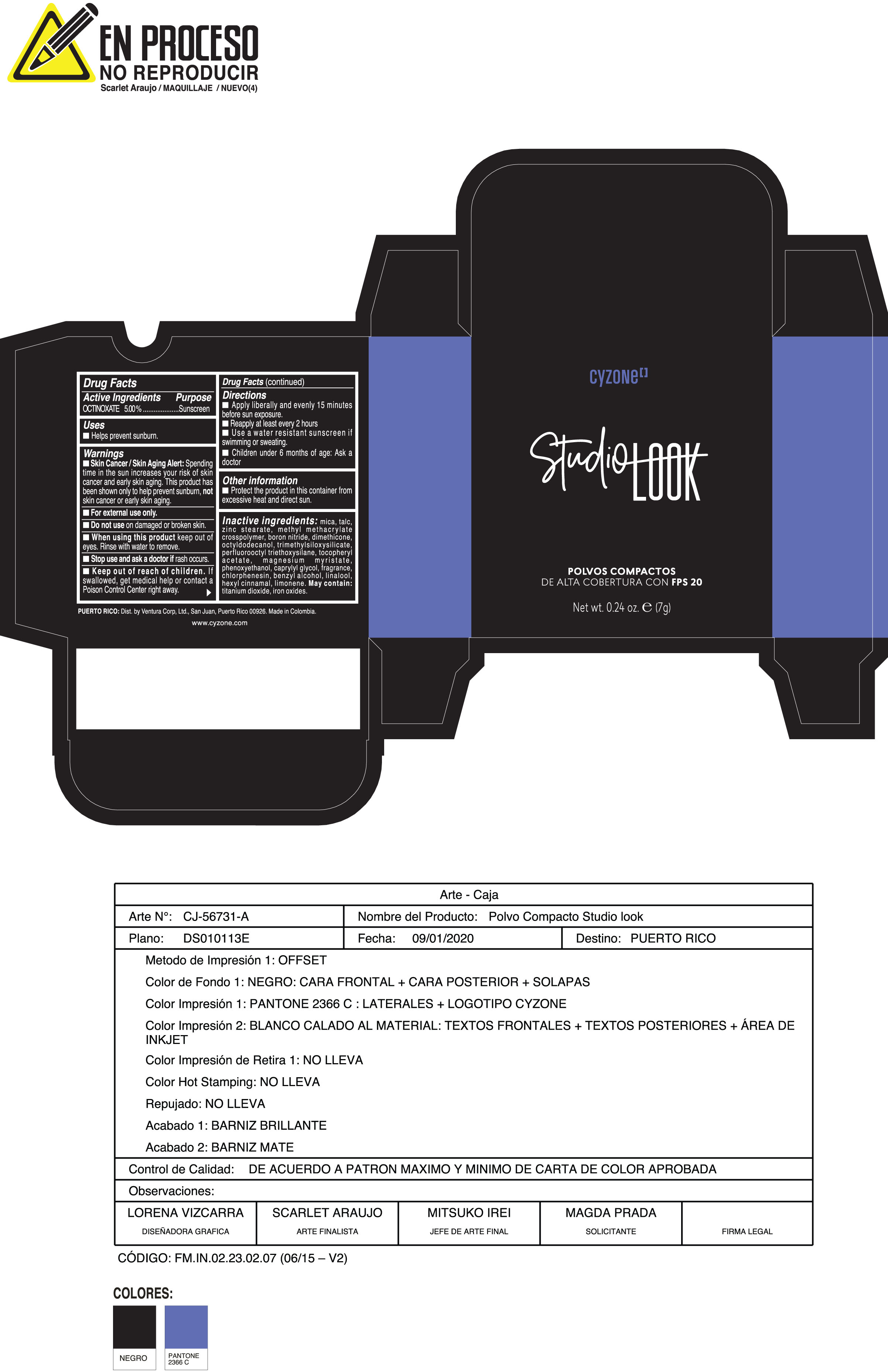
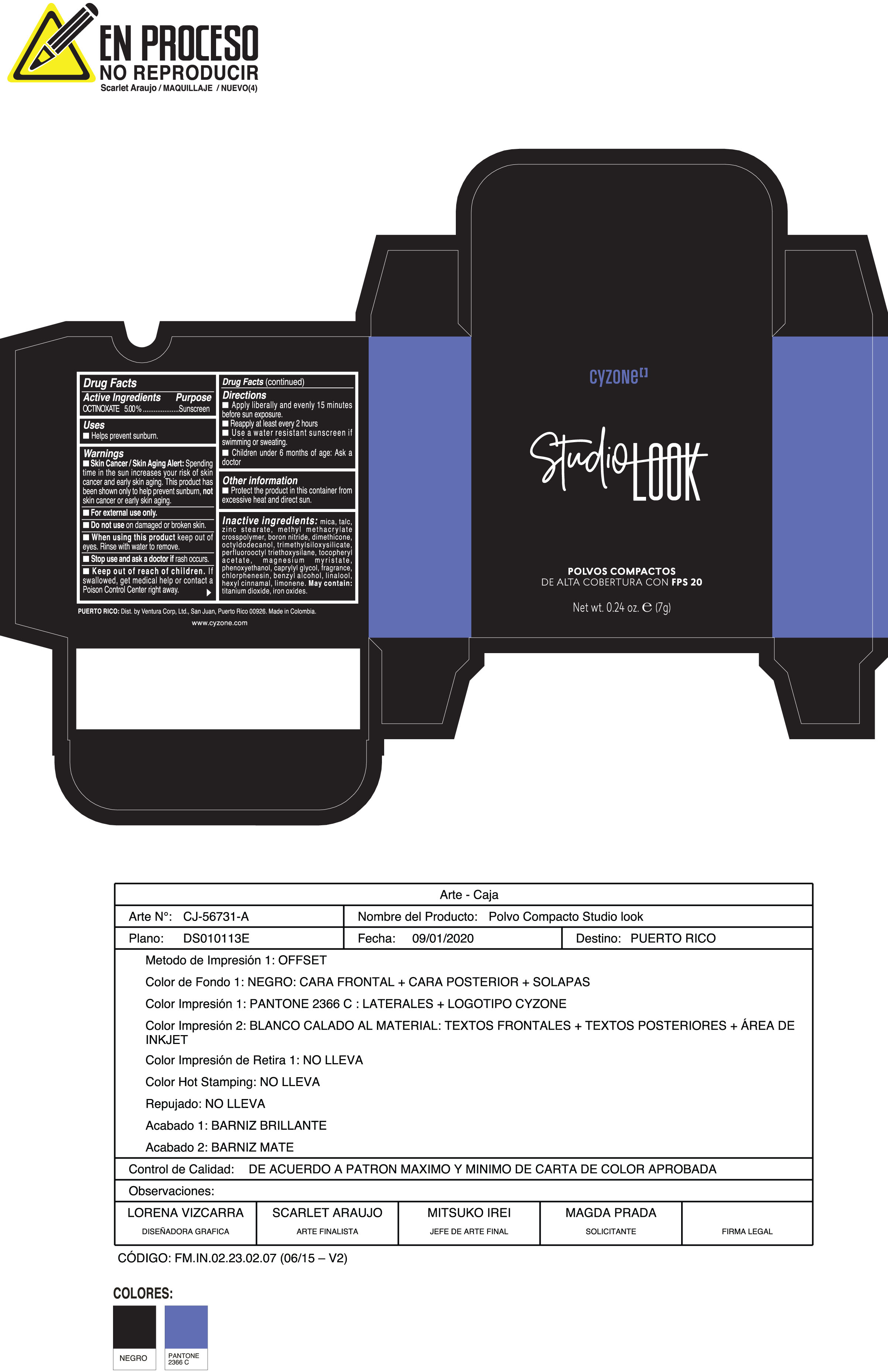
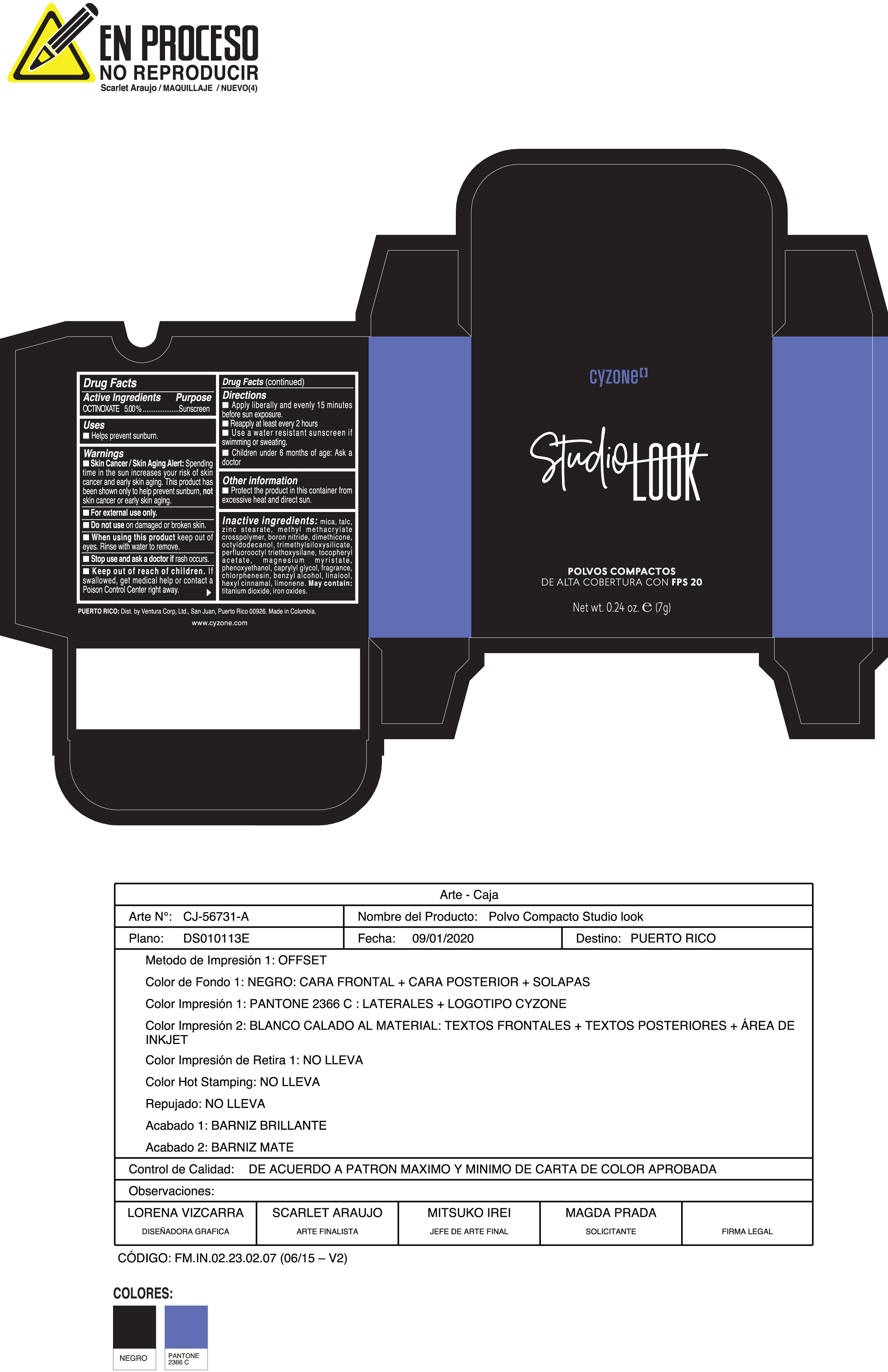
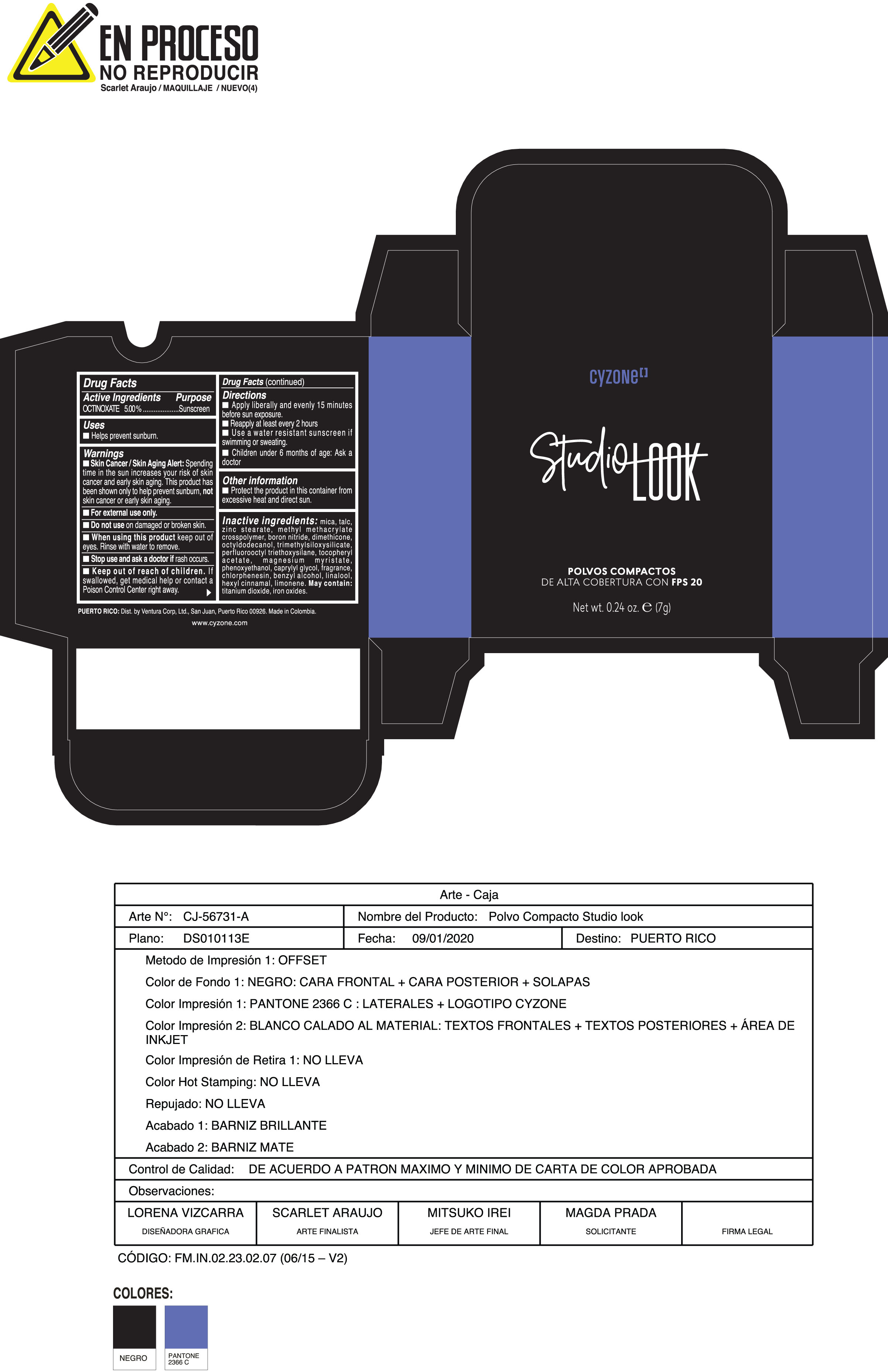
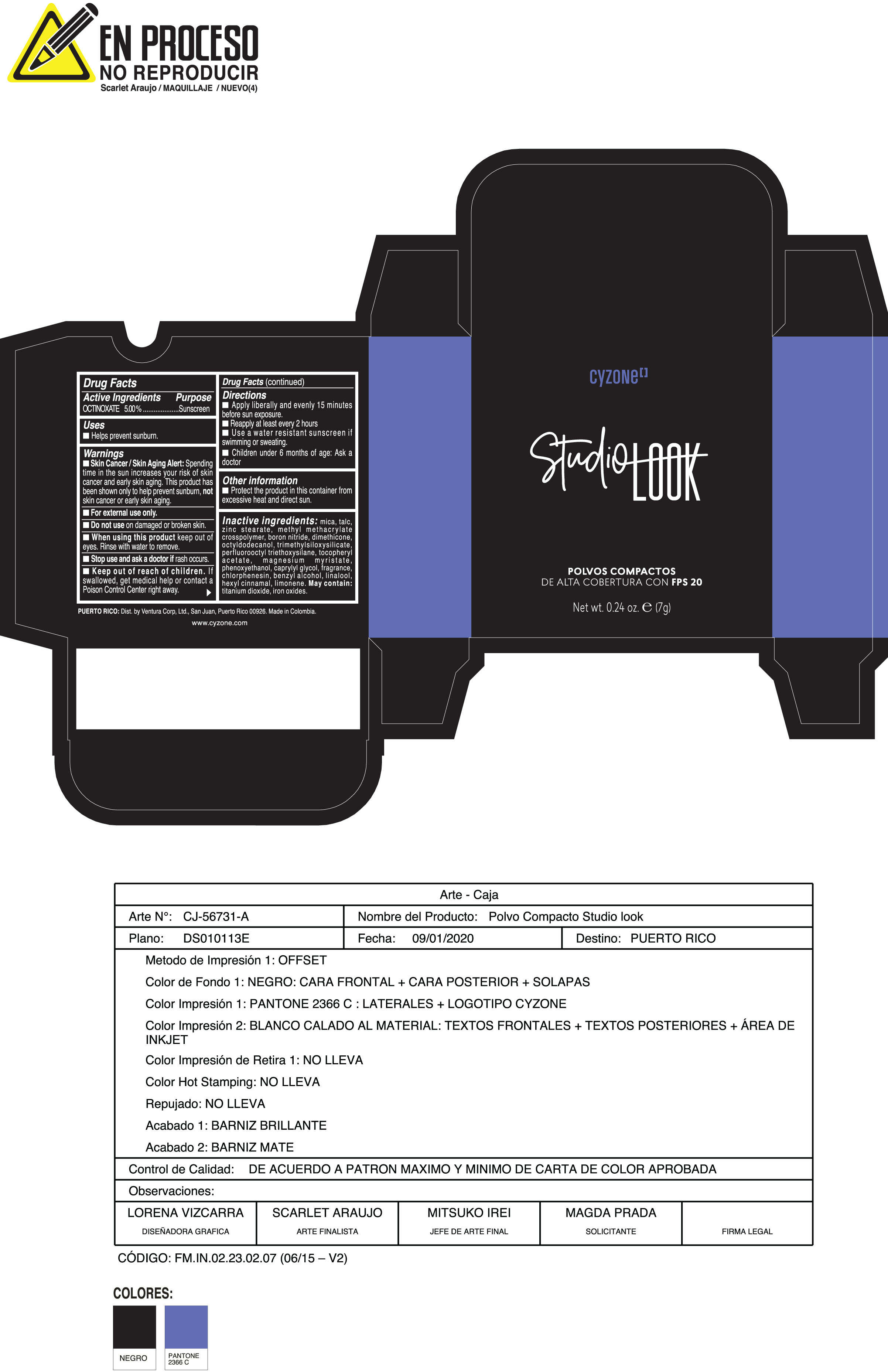
Label: POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 CREMA- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 FLAN- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 ALFAJOR- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 WAFFLE- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 COCADA- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 CAPUCCINO- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 MANJAR- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 MOCHA- octinoxate powder
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 COCOA- octinoxate powder
-
NDC Code(s):
14141-854-01,
14141-855-01,
14141-856-01,
14141-857-01, view more14141-858-01, 14141-859-01, 14141-860-01, 14141-861-01, 14141-862-01
- Packager: BEL STAR S A
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Skin Cancer/Skin Aging Alert:Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn notskin cancer or early skin aging.
Do not useon damaged or broken skin
When using this productkeep out of eyes. Rinse with water to remove
For external use only - DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients:mica, talc, zinc stearate, methyl methacrylate crosspolymer, boron nitride, dimethicone, octyldodecanol, trimethylsiloxysilicate, perfluorooctyl triethoxysilane, tocopheryl acetate, magnesium myristate, phenoxyethanol, caprylyl glycol, fragrance, chlorphenesin, benzyl alcohol, linalool, hexyl cinnamal, limonene. May Contain:titanium dioxide, iron oxides.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 CREMA
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-854 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) CHLORPHENESIN (UNII: I670DAL4SZ) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE (UNII: 92RU3N3Y1O) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) OCTYLDODECANOL (UNII: 461N1O614Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-854-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 02/09/2025 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 FLAN
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-855 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-855-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 02/08/2025 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 ALFAJOR
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-856 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-856-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 07/16/2024 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 WAFFLE
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-857 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-857-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 09/22/2024 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 COCADA
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-858 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-858-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 09/20/2024 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 CAPUCCINO
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-859 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-859-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 06/14/2025 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 MANJAR
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-860 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-860-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 03/15/2025 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 MOCHA
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-861 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-861-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 09/20/2024 POLVOS COMPACTOS DE ALTA COBERTURA CON FPS 20 COCOA
octinoxate powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-862 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FERRIC OXIDE RED (UNII: 1K09F3G675) BENZYL ALCOHOL (UNII: LKG8494WBH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIMETHYLSILOXYSILICATE (M/Q 1.0-1.2) (UNII: 78GX033D7I) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) ZINC STEARATE (UNII: H92E6QA4FV) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PERFLUOROOCTYL TRIETHOXYSILANE (UNII: 814P46684U) MAGNESIUM MYRISTATE (UNII: Z1917F0578) CAPRYLYL GLYCOL (UNII: 00YIU5438U) OCTYLDODECANOL (UNII: 461N1O614Y) MICA (UNII: V8A1AW0880) BORON NITRIDE (UNII: 2U4T60A6YD) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-862-01 7 g in 1 CARTON; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2020 09/21/2024 Labeler - BEL STAR S A (880160197) Establishment Name Address ID/FEI Business Operations BEL STAR S A 880160197 manufacture(14141-854, 14141-855, 14141-856, 14141-857, 14141-858, 14141-859, 14141-860, 14141-861, 14141-862)