Label: WALGREENS BOIL PAIN RELIEF- boil pain relief ointment
- NDC Code(s): 0363-9095-28
- Packager: Walgreens Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
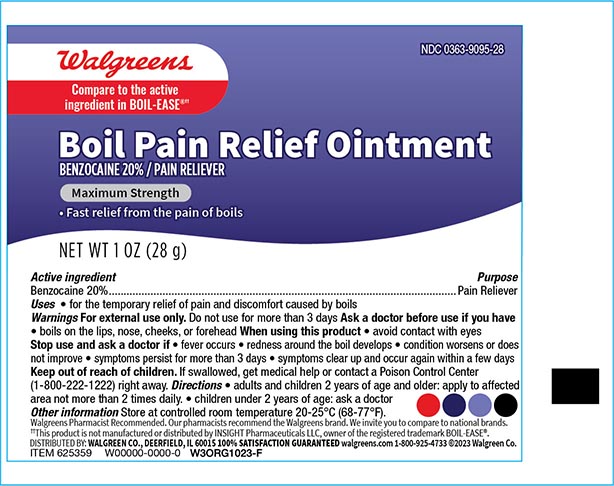
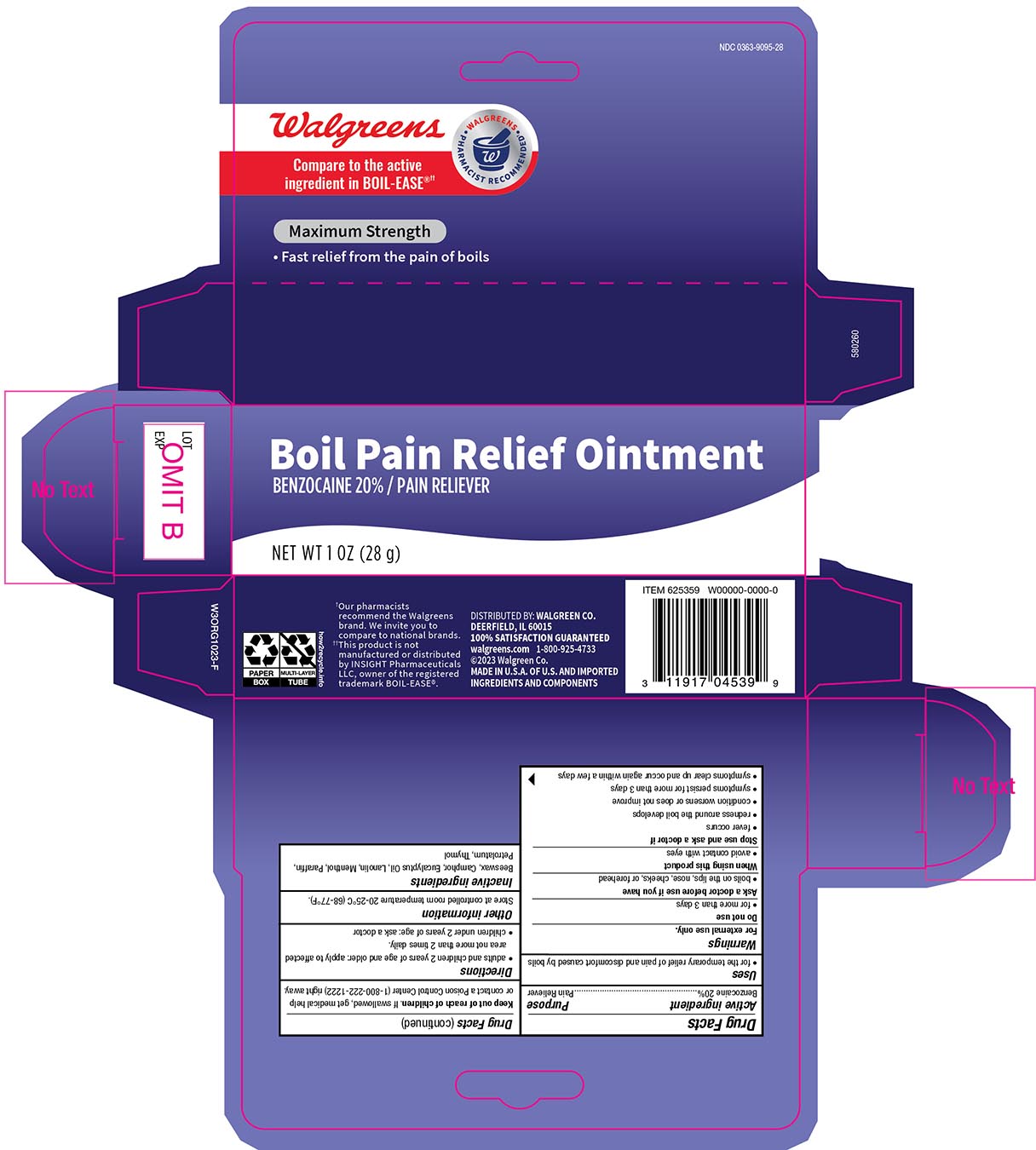
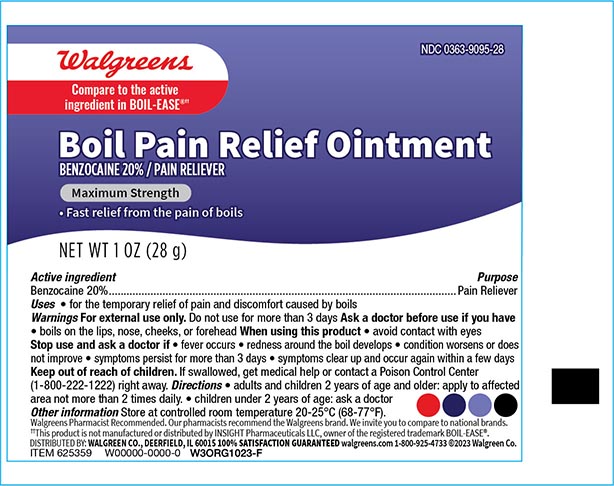
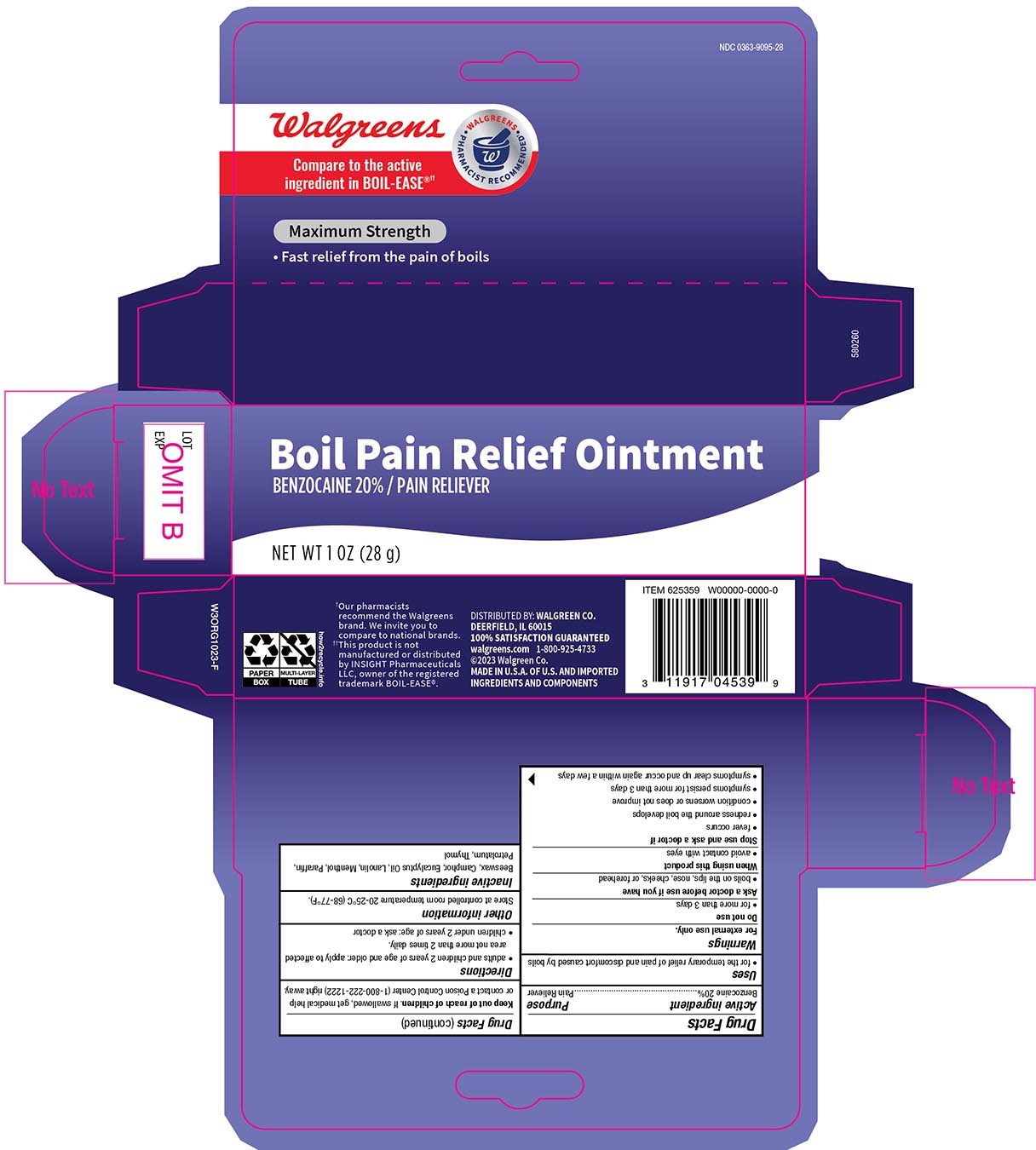
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- WarningsFor external use only
- Do not use
- When using this product
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive ingredients
- Principal display panel 1 oz
-
INGREDIENTS AND APPEARANCE
WALGREENS BOIL PAIN RELIEF
boil pain relief ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-9095 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) EUCALYPTUS OIL (UNII: 2R04ONI662) LANOLIN (UNII: 7EV65EAW6H) MENTHOL (UNII: L7T10EIP3A) PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) THYMOL (UNII: 3J50XA376E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-9095-28 1 in 1 CARTON 02/01/2024 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/01/2024 Labeler - Walgreens Company (008965063)