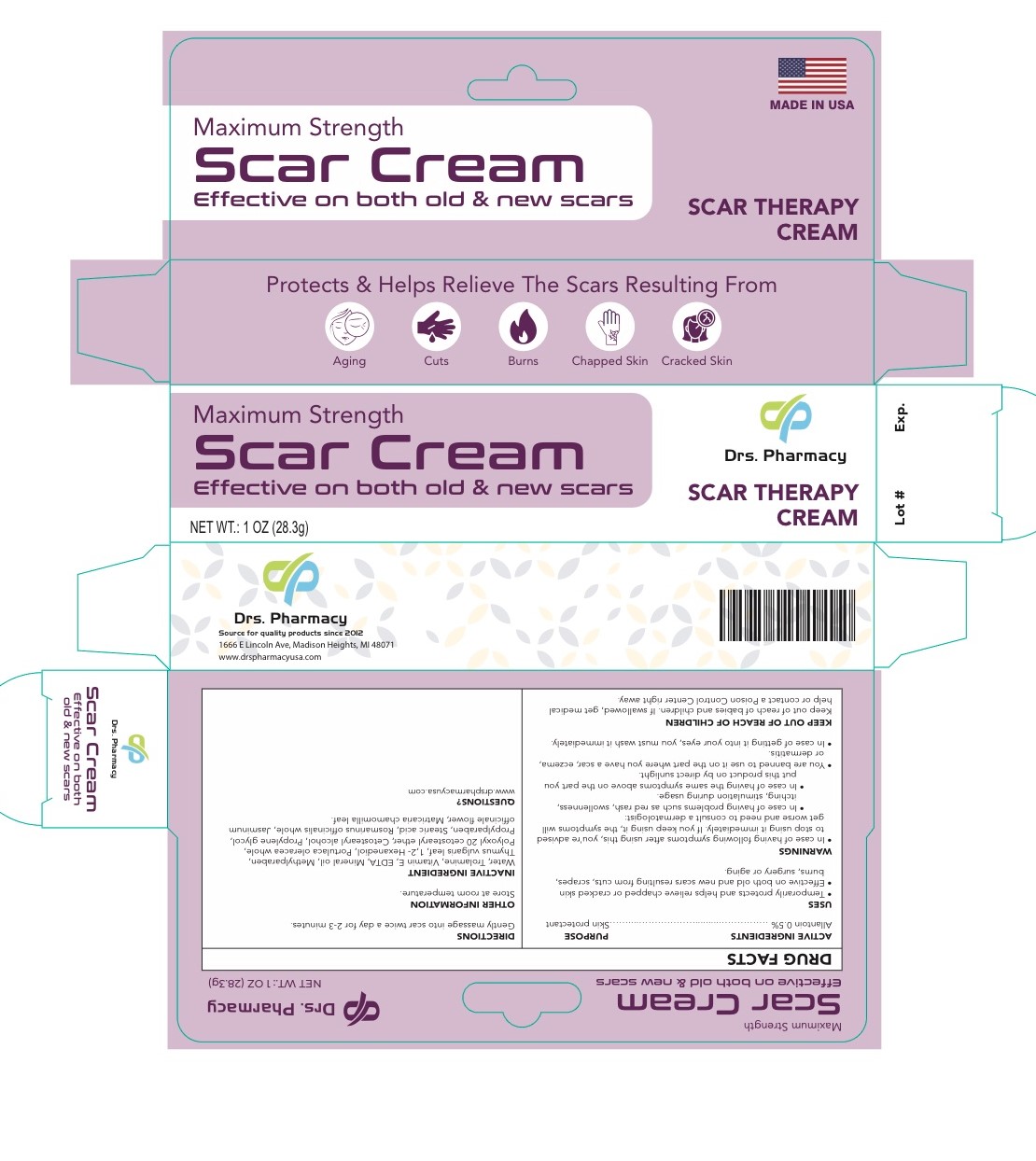
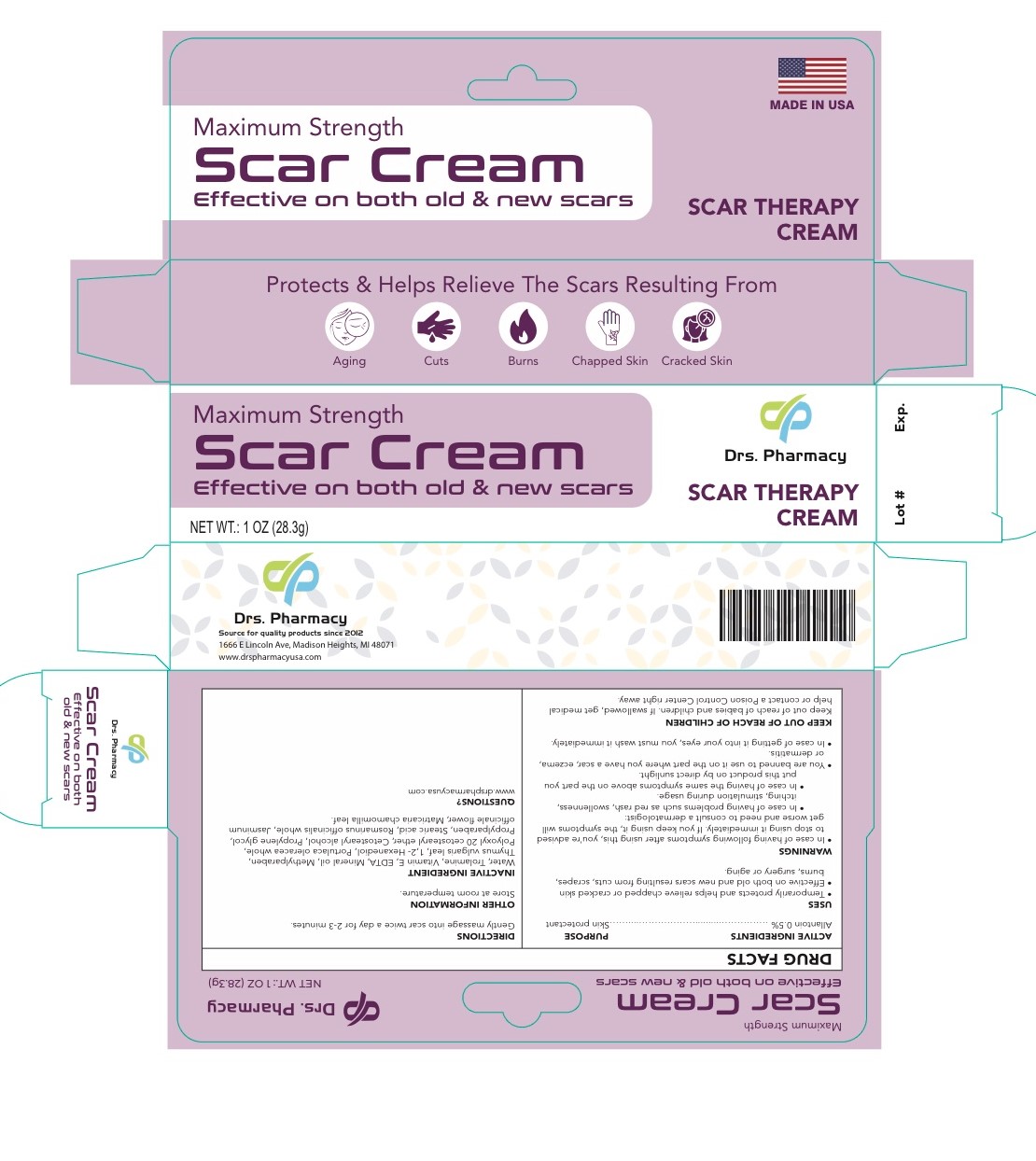
Label: DRS. PHARMACY SCAR CREAM- allantoin cream
- NDC Code(s): 80489-333-01, 80489-333-02
- Packager: OL PHARMA TECH, LLC Drs PHARMACY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PURPOSE
- INDICATIONS & USAGE
-
Inactive Ingredients
Cetostearyl alcohol- mineral oil- ceteareth 20- propylene glycol- methyl paraben- propylparaben- vitamin E- Stearic acid - trolamine RosmarinusOfficinalis (Rosemary)Extract/ Portulaca Oleracea Extract/Thymus Vulgaris Thyme) Leaf Extract/Jasminum Officinale (jasmine) Flower/ Leaf Extract?Chamomila) Recutita (Matricaria) Flower Water, Aureobasidium Pullulans Ferment Extract/ 1.2-Hexanedio
-
Warnings
- In case of having the following symptoms after using this cream, you are advised to stop using it immediately. If you keep using it , the symptoms will get worse and need to consult a drematologist .1- in case of having problems such as red rash, swollllenness, itching, stimulation during usage. 2- in case of having the same symptoms above on the part you put this product on by direct sunlight
- you are banned to use it on the part where you have a eczema, lor dermatitis.
- in case of getting it into your eyes, you have to wash it immediately.
- Questions
- KEEP OUT OF REACH OF CHILDREN
- package label
-
INGREDIENTS AND APPEARANCE
DRS. PHARMACY SCAR CREAM
allantoin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80489-333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) EDETATE DISODIUM (UNII: 7FLD91C86K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) MINERAL OIL (UNII: T5L8T28FGP) METHYLPARABEN (UNII: A2I8C7HI9T) THYMUS VULGARIS LEAF (UNII: GRX3499643) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) MATRICARIA CHAMOMILLA LEAF (UNII: 6I9LN466F0) ROSMARINUS OFFICINALIS WHOLE (UNII: EA3289138M) PORTULACA OLERACEA WHOLE (UNII: D5J3623SV2) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80489-333-01 1 in 1 CARTON 11/01/2021 1 20 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:80489-333-02 1 in 1 CARTON 11/01/2021 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/01/2021 Labeler - OL PHARMA TECH, LLC Drs PHARMACY (021170377) Establishment Name Address ID/FEI Business Operations OL PHARMA TECH, LLC Drs PHARMACY 021170377 manufacture(80489-333)