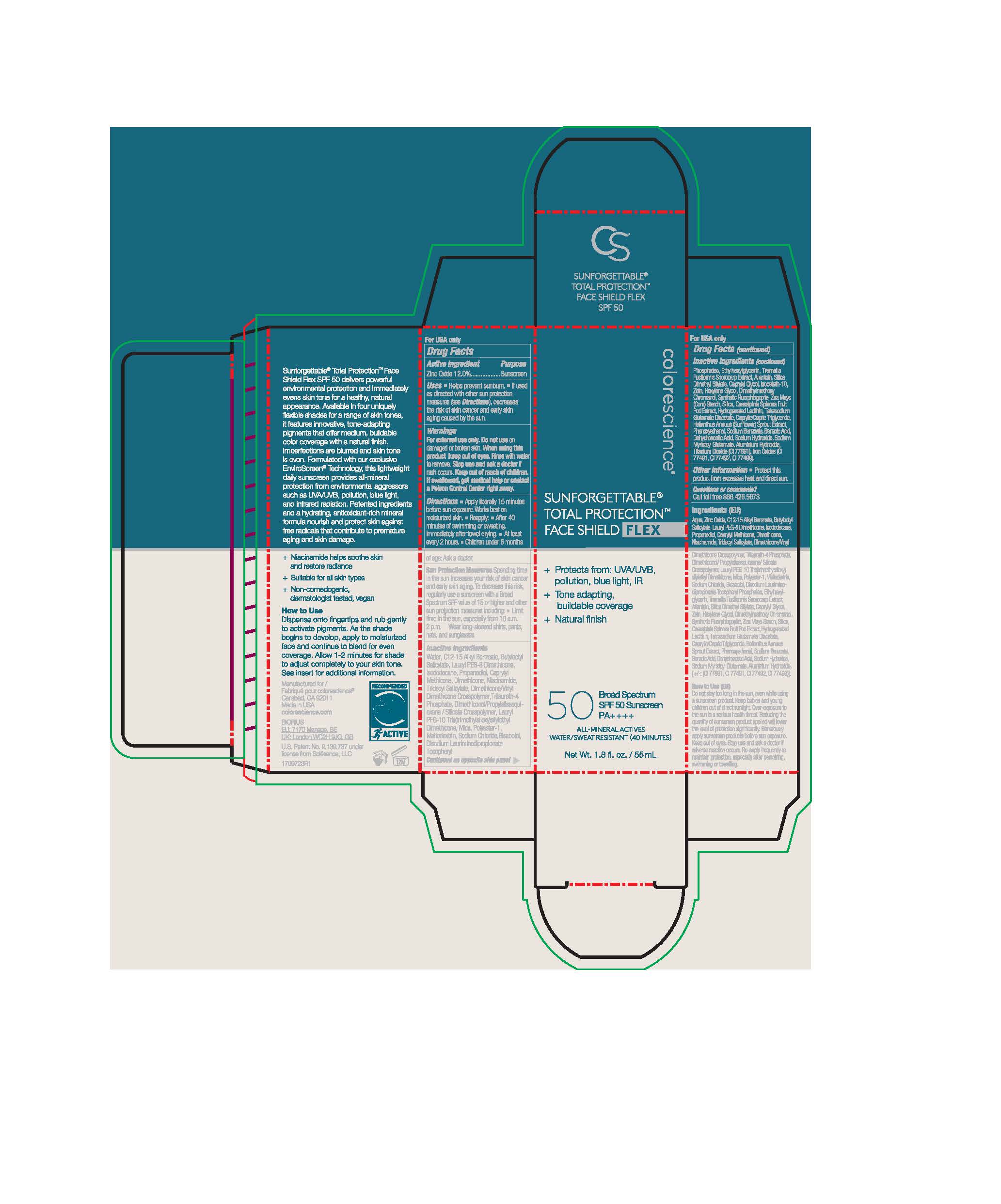
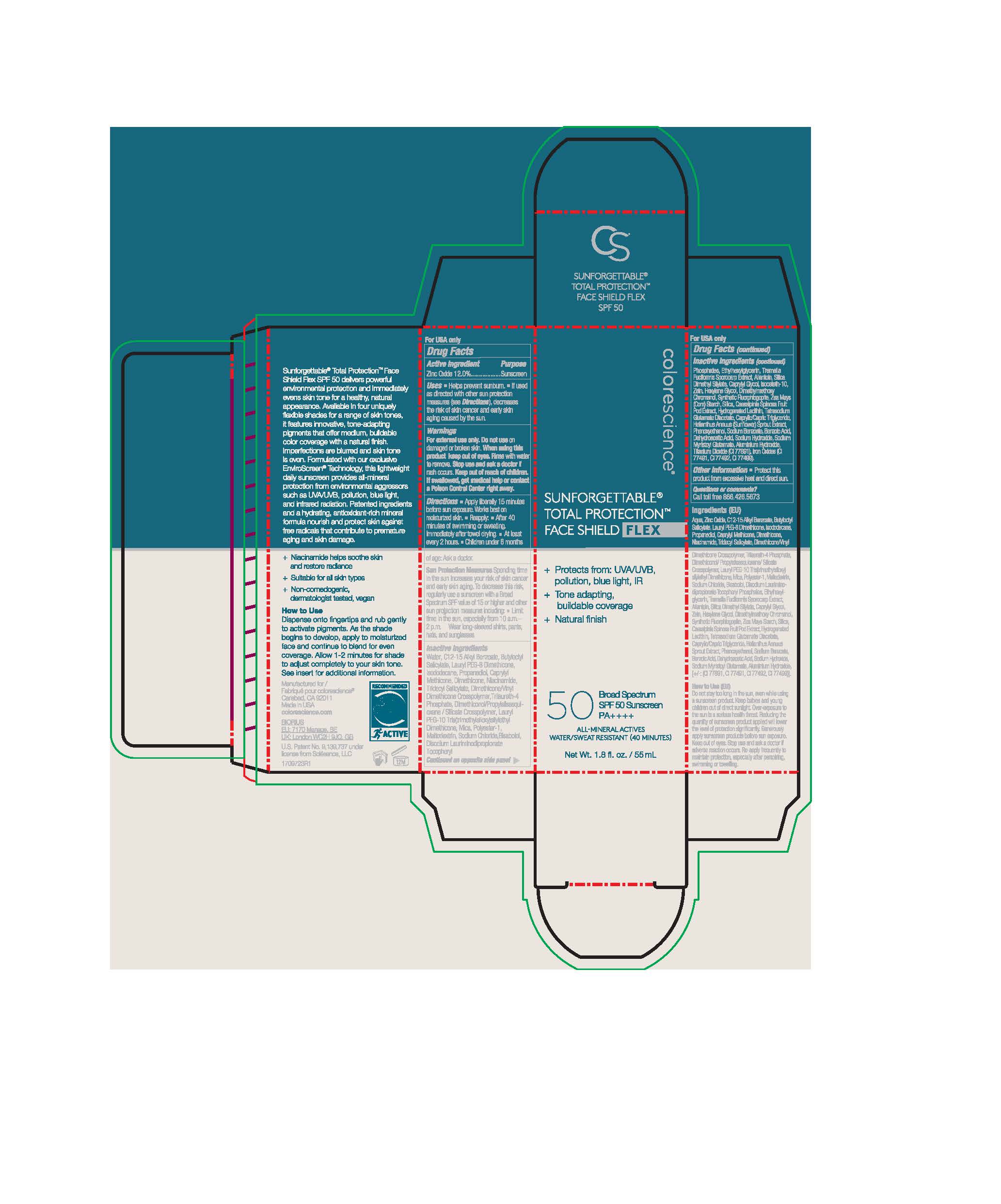
Label: SUNFORGETTABLE TOTAL PROTECTION FACE SHIELD FLEX- zinc oxide lotion
- NDC Code(s): 68078-042-01, 68078-042-02
- Packager: Nanophase Technologies Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Sunforgettable Total Protection Face Shield Flex
-
INGREDIENTS AND APPEARANCE
SUNFORGETTABLE TOTAL PROTECTION FACE SHIELD FLEX
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-042 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 135.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM MYRISTOYL GLUTAMATE (UNII: AYU7QD893W) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) DIMETHYLMETHOXY CHROMANOL (UNII: XBH432G01F) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MYRISTYL TRISILOXANE (UNII: J7960S4R1T) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-BISABOLOL, (+)- (UNII: 105S6I733Z) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) TRILAURETH-4 PHOSPHATE (UNII: M96W2OLL2V) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ISODODECANE (UNII: A8289P68Y2) NIACINAMIDE (UNII: 25X51I8RD4) WATER (UNII: 059QF0KO0R) DIMETHICONOL/PROPYLSILSESQUIOXANE/SILICATE CROSSPOLYMER (450000000 MW) (UNII: 9KB5R958PB) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) DEHYDROACETIC ACID (UNII: 2KAG279R6R) PROPANEDIOL (UNII: 5965N8W85T) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) SODIUM CHLORIDE (UNII: 451W47IQ8X) PHENOXYETHANOL (UNII: HIE492ZZ3T) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) MALTODEXTRIN (UNII: 7CVR7L4A2D) DIMETHICONE (UNII: 92RU3N3Y1O) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DISODIUM LAURIMINODIPROPIONATE TOCOPHERYL PHOSPHATES (UNII: 0K5Y9U1P6M) ISOCETETH-10 (UNII: 1K92T9919H) CAESALPINIA SPINOSA FRUIT POD (UNII: EXY4496LWD) HELIANTHUS ANNUUS SPROUT (UNII: 4P26HG1S5W) MICA (UNII: V8A1AW0880) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) ZEIN (UNII: 80N308T1NN) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-042-02 1 in 1 CARTON 05/06/2021 1 NDC:68078-042-01 55 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/06/2021 Labeler - Nanophase Technologies Corporation (128731929) Registrant - Nanophase Technologies Corporation (623502044) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 050383046 api manufacture(68078-042) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 623502044 api manufacture(68078-042) , manufacture(68078-042) Establishment Name Address ID/FEI Business Operations Nanophase Technologies Corporation 118812921 pack(68078-042) , manufacture(68078-042)