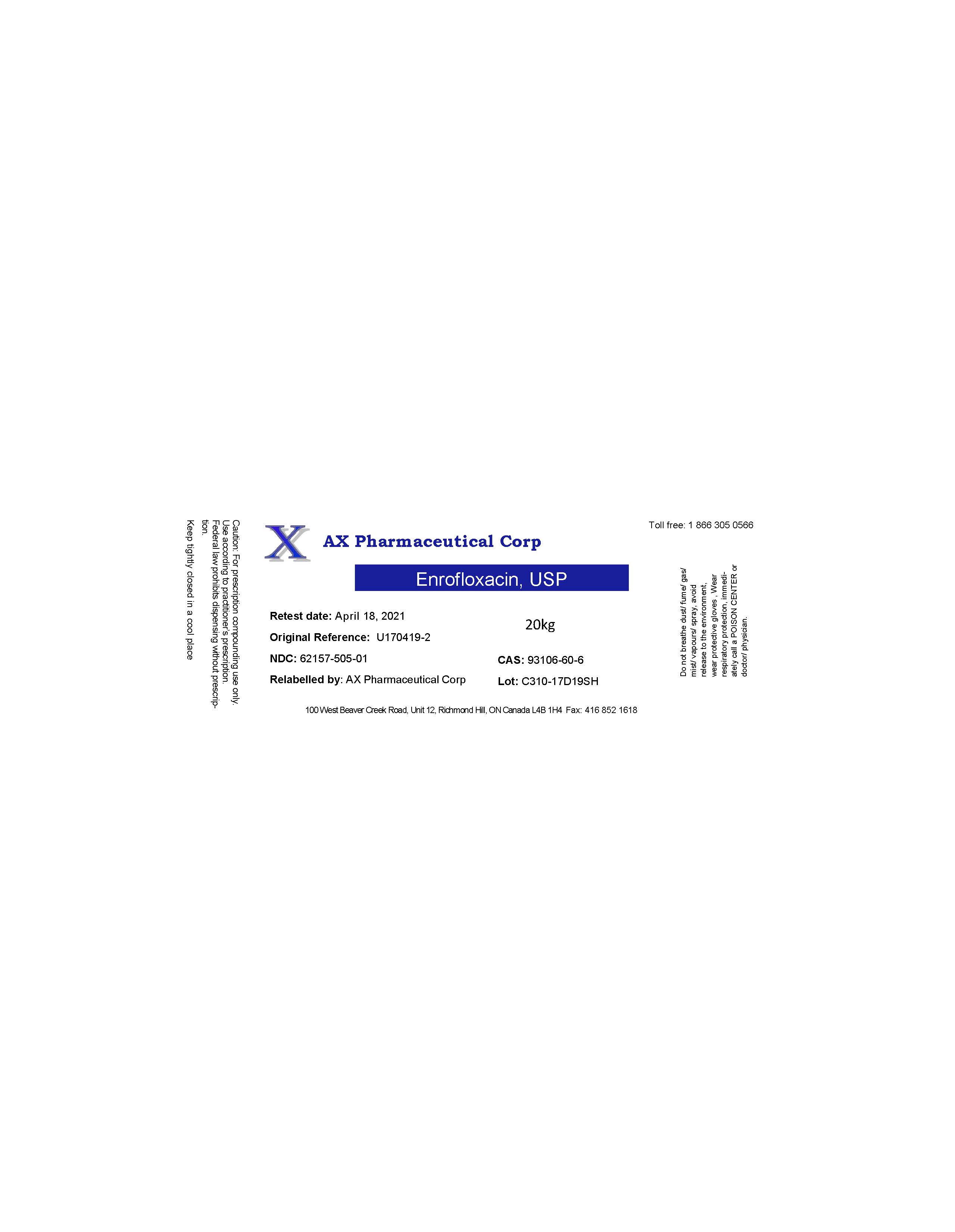
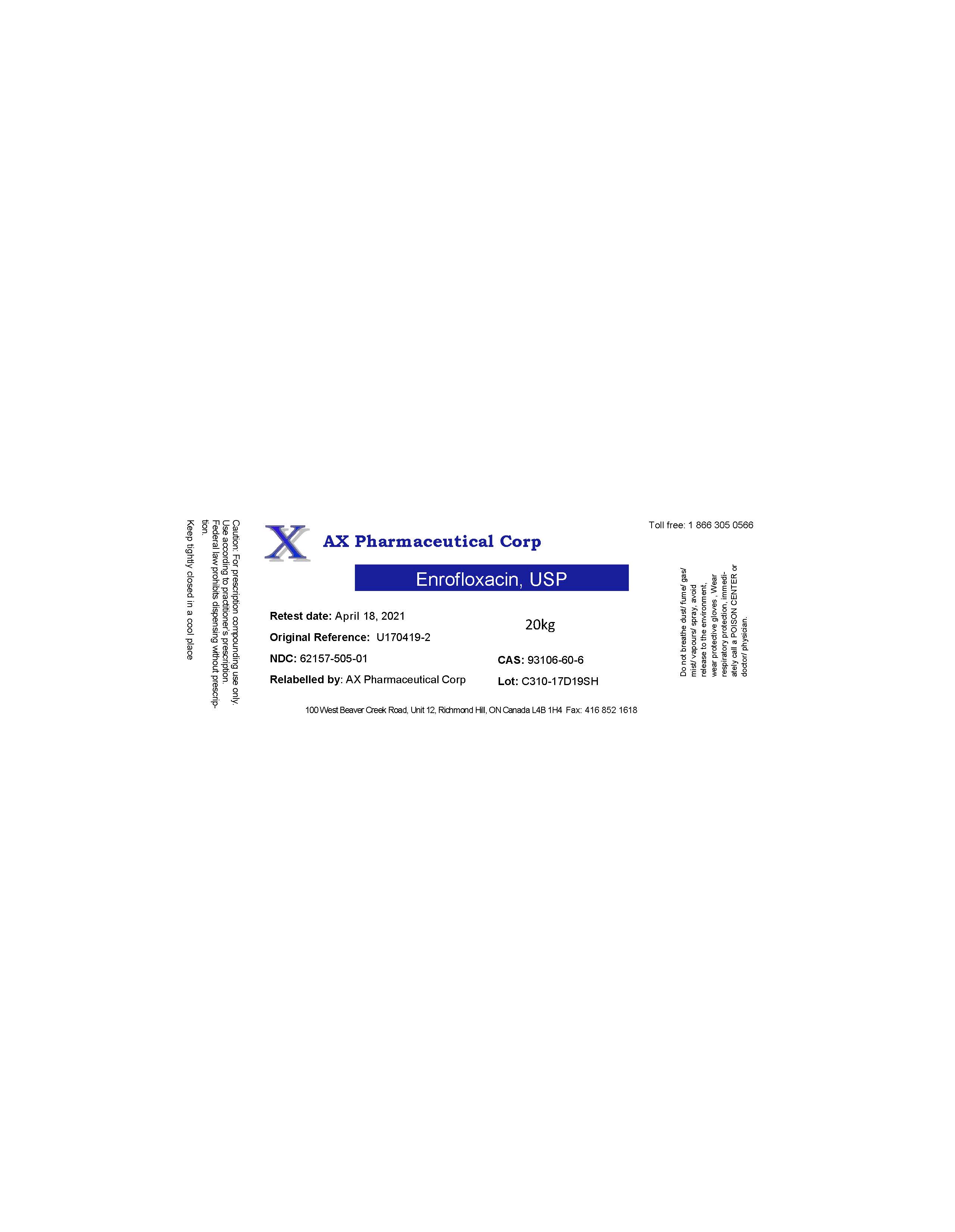
Label: AX PHARMACEUTICAL CORP- enrofloxacin powder
- NDC Code(s): 62157-505-01
- Packager: AX Pharmaceutical Corp
- Category: BULK INGREDIENT
Drug Label Information
Updated December 8, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AX PHARMACEUTICAL CORP
enrofloxacin powderProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:62157-505 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENROFLOXACIN (UNII: 3DX3XEK1BN) (ENROFLOXACIN - UNII:3DX3XEK1BN) ENROFLOXACIN 19.8 kg in 20 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62157-505-01 20 kg in 1 DRUM 12/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient for animal drug compounding 12/08/2017 Labeler - AX Pharmaceutical Corp (202924858)