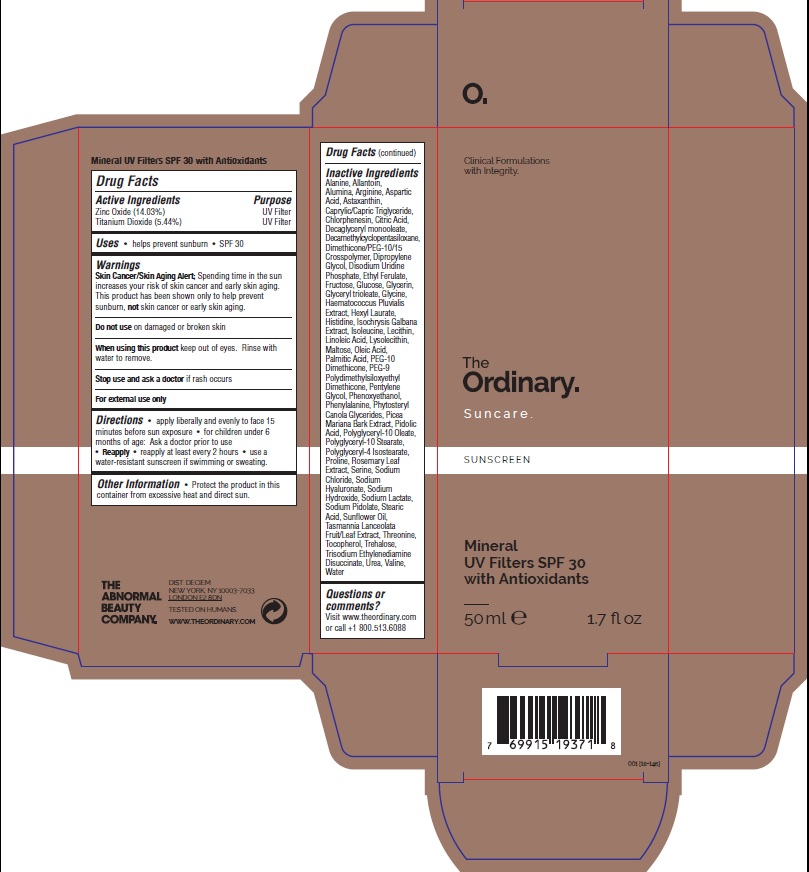
Label: THE ORDINARY SUNCARE MINERAL UV FILTERS WITH ANTIOXIDANTS SPF30- zinc oxide, titanium dioxid lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 72208-352-01, 72208-352-04 - Packager: Deciem Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acrtive Ingredients
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water, Cyclopentasiloxane, PEG-10 Dimethicone, Glycerin, Helianthus Annuus Seed Oil, PEG-9 Polydimethylsiloxyethyl Dimethicone, Hexyl Laurate, Polyglyceryl-4 Isostearate, Caprylic/Capric Triglyceride, Dimethicone/PEG-10/15 Crosspolymer, Astaxanthin, Disodium Uridine Phosphate, Ethyl Ferulate, Rosmarinus Officinalis Leaf Extract, Tasmannia Lanceolata Fruit/Leaf Extract, Haematococcus Pluvialis Extract, Picea Mariana Bark Extract, Arginine, Aspartic Acid, Glycine, Alanine, Serine, Valine, Isoleucine, Proline, Threonine, Histidine, Phenylalanine, Sodium PCA, PCA, Sodium Lactate, Glucose, Maltose, Fructose, Trehalose, Urea, Allantoin, Sodium Hyaluronate, Linoleic Acid, Oleic Acid, Phytosteryl Canola Glycerides, Palmitic Acid, Stearic Acid, Isochrysis Galbana Extract, Lysolecithin, Lecithin, Triolein, Pentylene Glycol, Dipropylene Glycol, Polyglyceryl-10 Oleate, Polyglyceryl-5 Trioleate, Polyglyceryl-10 Stearate, Tocopherol, Alumina, Citric Acid, Trisodium Ethylenediamine Disuccinate, Sodium Chloride, Sodium Hydroxide, Phenoxyethanol, Chlorphenesin.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THE ORDINARY SUNCARE MINERAL UV FILTERS WITH ANTIOXIDANTS SPF30
zinc oxide, titanium dioxid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72208-352 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 14.03 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.44 g in 100 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) GLYCERIN (UNII: PDC6A3C0OX) SUNFLOWER OIL (UNII: 3W1JG795YI) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) HEXYL LAURATE (UNII: 4CG9F9W01Q) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ASTAXANTHIN (UNII: 8XPW32PR7I) URIDINE MONOPHOSPHATE DISODIUM (UNII: KD8E20071T) ETHYL FERULATE (UNII: 5B8915UELW) ROSEMARY (UNII: IJ67X351P9) TASMANNIA LANCEOLATA WHOLE (UNII: RQ58K8571Z) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) PICEA MARIANA WHOLE (UNII: U37W7O088B) ARGININE (UNII: 94ZLA3W45F) ASPARTIC ACID (UNII: 30KYC7MIAI) GLYCINE (UNII: TE7660XO1C) ALANINE (UNII: OF5P57N2ZX) SERINE (UNII: 452VLY9402) VALINE (UNII: HG18B9YRS7) ISOLEUCINE (UNII: 04Y7590D77) PROLINE (UNII: 9DLQ4CIU6V) THREONINE (UNII: 2ZD004190S) HISTIDINE (UNII: 4QD397987E) PHENYLALANINE (UNII: 47E5O17Y3R) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PIDOLIC ACID (UNII: SZB83O1W42) SODIUM LACTATE (UNII: TU7HW0W0QT) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MALTOSE (UNII: XJ6S9RV06F) FRUCTOSE (UNII: 6YSS42VSEV) TREHALOSE (UNII: B8WCK70T7I) UREA (UNII: 8W8T17847W) ALLANTOIN (UNII: 344S277G0Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LINOLEIC ACID (UNII: 9KJL21T0QJ) OLEIC ACID (UNII: 2UMI9U37CP) CANOLA OIL (UNII: 331KBJ17RK) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) GLYCERYL TRIOLEATE (UNII: O05EC62663) PENTYLENE GLYCOL (UNII: 50C1307PZG) DIPROPYLENE GLYCOL (UNII: E107L85C40) POLYGLYCERYL-10 OLEATE (UNII: 55C81W76DH) POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) TOCOPHEROL (UNII: R0ZB2556P8) ALUMINUM OXIDE (UNII: LMI26O6933) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72208-352-04 1 in 1 CARTON 05/18/2018 1 NDC:72208-352-01 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/18/2018 Labeler - Deciem Inc (203133665) Establishment Name Address ID/FEI Business Operations Crystal Claire Cosmetics Inc. 205493484 manufacture(72208-352) , pack(72208-352)