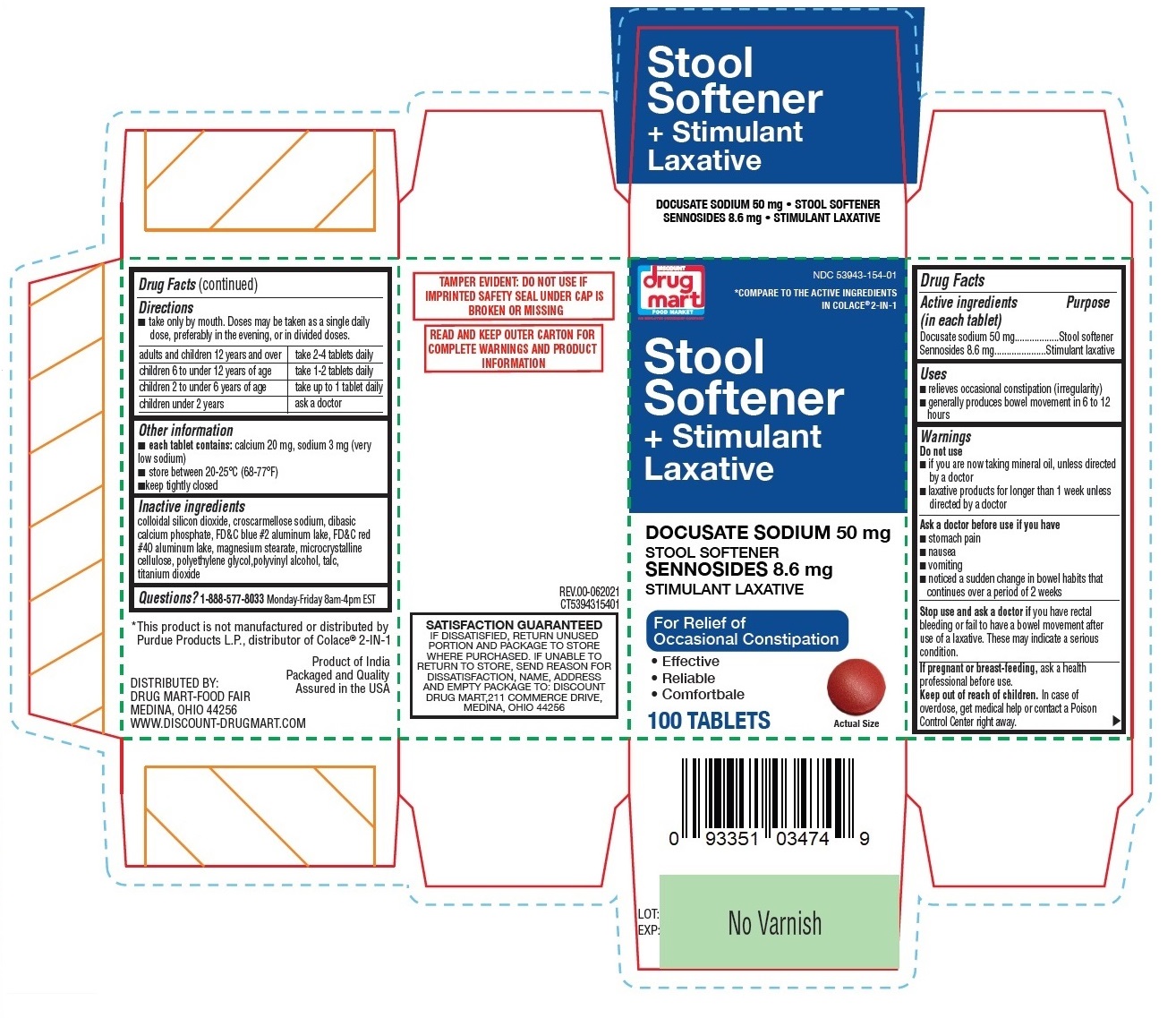
Label: DRUG MART STOOL SOFTENER PLUS STIMULANT LAXATIVE- docusate sodium, sennosides tablet
- NDC Code(s): 53943-154-01
- Packager: Discount Drug Mart, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 27, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
- take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
adults and children 12 years and over take 2-4 tablets daily children 6 to under 12 years of age take 1-2 tablets daily children 2 to under 6 years of age take up to 1 tablet daily children under 2 years ask a doctor - Other information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
COMPARE TO THE ACTIVE INGREDIENTS IN COLACE®2-IN-1
For Relief of Occasional Constipation
- Effective
- Reliable
- Comfortable
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
READ AND KEEP OUTER CARTON FOR COMPLETE PRODUCT INFORMATION
SATISFACTION GUARANTEED
IF DISSATISFIED, RETURN UNUSED PORTION AND PACKAGE TO STORE WHERE PURCHASED. IF UNABLE TO RETURN TO STORE, SEND REASON FOR DISSATISFACTION, NAME, ADDRESS AND EMPTY PACKAGE TO: DISCOUNT DRUG MART,211 COMMERCE DRIVE, MEDINA, OHIO 44256* This product is not manufactured or distributed by Purdue Products L.P., distributor of Colace®2-IN-1.
DISTRIBUTED BY:
DRUG MART-FOOD FAIR
MEDINA, OHIO 44256
WWW.DISCOUNT-DRUGMART.COMProduct of India
Packaged and Quality Assured in the USA - Packaging
-
INGREDIENTS AND APPEARANCE
DRUG MART STOOL SOFTENER PLUS STIMULANT LAXATIVE
docusate sodium, sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-154 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 10mm Flavor Imprint Code S154 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-154-01 1 in 1 CARTON 07/20/2021 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 07/20/2021 Labeler - Discount Drug Mart, Inc (047741335) Registrant - Strive Pharmaceuticals Inc. (080028013)