Label: KROGER SALINE- nasal spray
- NDC Code(s): 41226-759-30
- Packager: KROGER COMPANY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug FactsActive Ingredients
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions or comments?
-
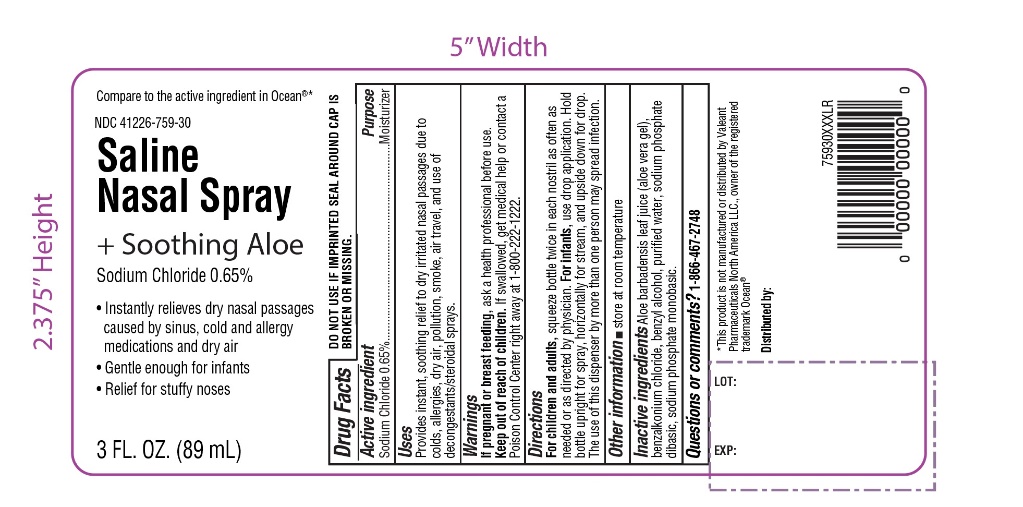
Saline Nasal Spray
Compare to the active ingredient in Ocean®*
NDC: 41226-759-30
Saline Nasal Spray
+ Soothing Aloe
Sodium Chloride 0.65%
- •
- Instantly relieves dry nasal passages caused by sinus, cold and allergy medications and dry air
- •
- Gentle enough for infants
- •
- Relief for stuffy noses
3 FL O.Z. (89 mL)
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL AROUND CAP IS BROKEN OR MISSING.
Distributed by:
-
INGREDIENTS AND APPEARANCE
KROGER SALINE
nasal sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41226-759 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41226-759-30 1 in 1 CARTON 01/02/2024 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/02/2024 Labeler - KROGER COMPANY (006999528)