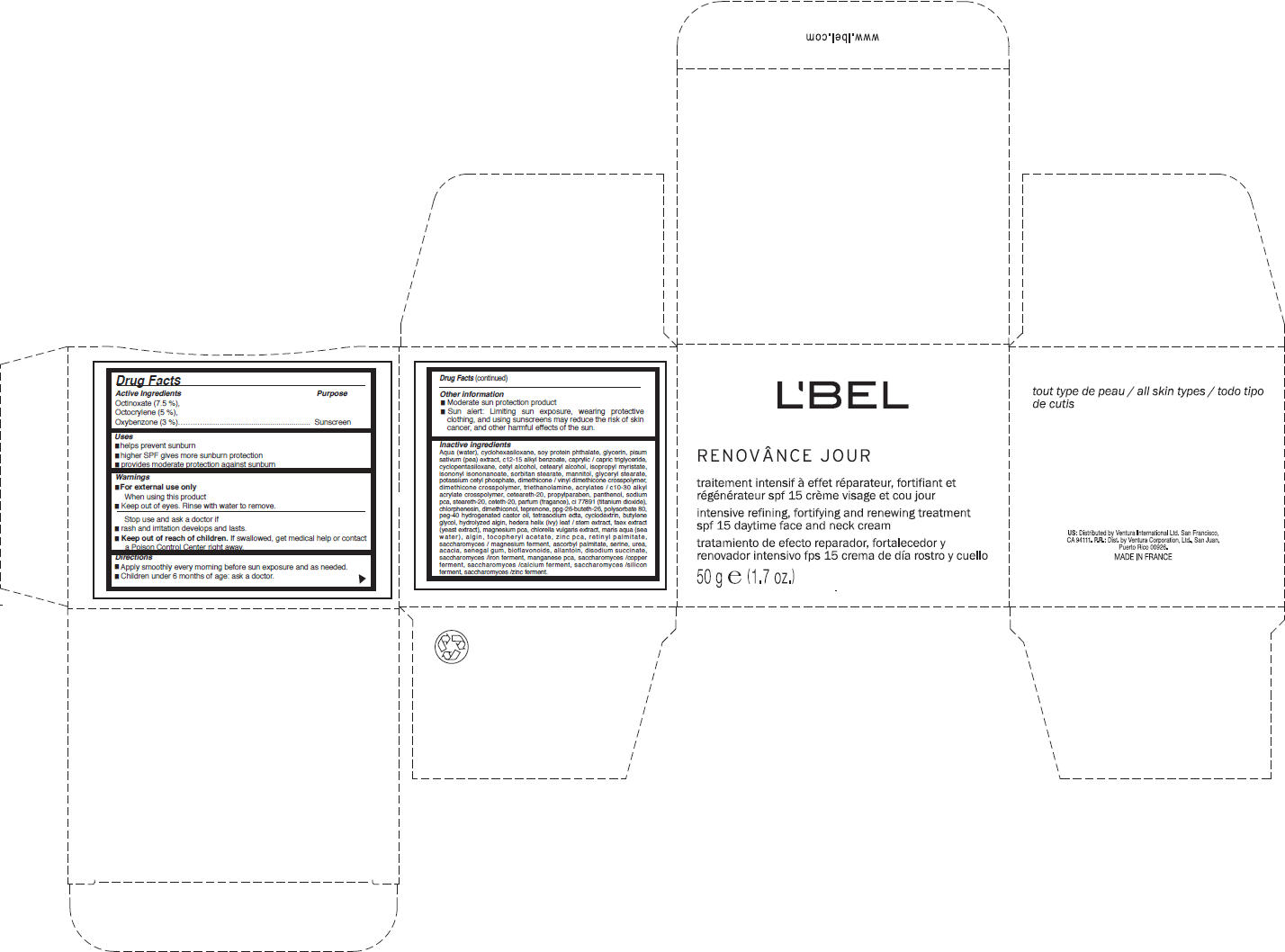
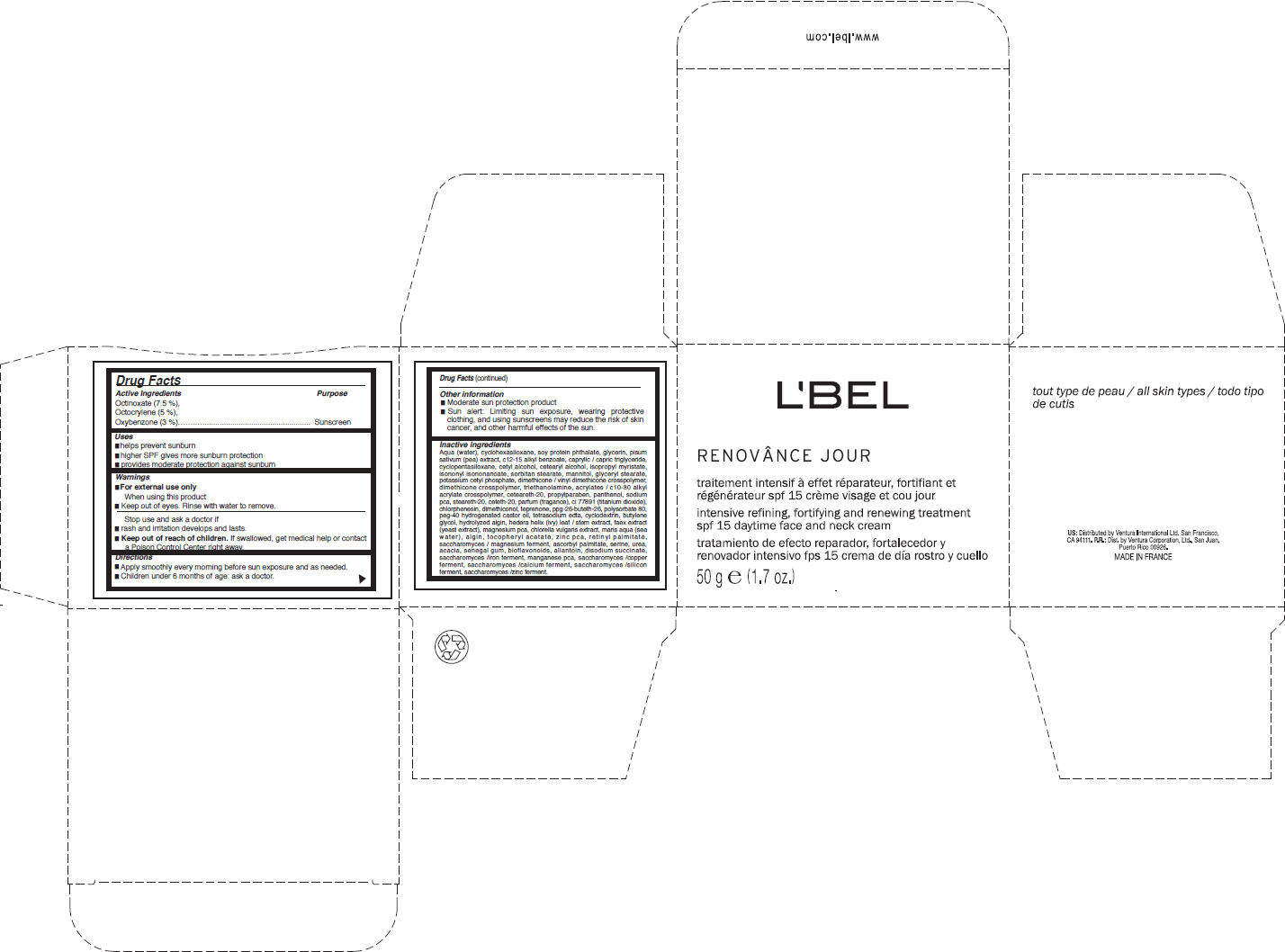
Label: LBEL PARIS- octinoxate, octocrylene, and oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 14783-018-41, 14783-018-42, 14783-028-51, 14783-028-52, view more14783-038-61, 14783-038-62 - Packager: Ventura International LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 10, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (water), cyclohexasiloxane, soy protein phthalate, glycerin, pisum sativum (pea) extract, c12-15 alkyl benzoate, caprylic / capric triglyceride, cyclopentasiloxane, cetyl alcohol, cetearyl alcohol, isopropyl myristate, isononyl isononanoate, sorbitan stearate, mannitol, glyceryl stearate, potassium cetyl phosphate, dimethicone / vinyl dimethicone crosspolymer, dimethicone crosspolymer, triethanolamine, acrylates / c10-30 alkyl acrylate crosspolymer, ceteareth-20, propylparaben, panthenol, sodium pca, steareth-20, ceteth-20, parfum (fragance), ci 77891 (titanium dioxide), chlorphenesin, dimethiconol, teprenone, ppg-26-buteth-26, polysorbate 80, peg-40 hydrogenated castor oil, tetrasodium edta, cyclodextrin, butylene glycol, hydrolyzed algin, hedera helix (ivy) leaf / stem extract, faex extract (yeast extract), magnesium pca, chlorella vulgaris extract, maris aqua (sea water), algin, tocopheryl acetate, zinc pca, retinyl palmitate, saccharomyces / magnesium ferment, ascorbyl palmitate, serine, urea, acacia, senegal gum, bioflavonoids, allantoin, disodium succinate, saccharomyces /iron ferment, manganese pca, saccharomyces /copper ferment, saccharomyces /calcium ferment, saccharomyces /silicon ferment, saccharomyces /zinc ferment.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 g Jar Carton
-
INGREDIENTS AND APPEARANCE
LBEL PARIS RENOVANCE JOUR
octinoxate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 3.75 g in 50 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 2.5 g in 50 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 1.5 g in 50 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) cyclomethicone 6 (UNII: XHK3U310BA) glycerin (UNII: PDC6A3C0OX) snow pea (UNII: 84SKC33B1I) c12-15 alkyl benzoate (UNII: A9EJ3J61HQ) medium-chain triglycerides (UNII: C9H2L21V7U) cyclomethicone 5 (UNII: 0THT5PCI0R) cetyl alcohol (UNII: 936JST6JCN) cetostearyl alcohol (UNII: 2DMT128M1S) isopropyl myristate (UNII: 0RE8K4LNJS) isononyl isononanoate (UNII: S4V5BS6GCX) sorbitan monostearate (UNII: NVZ4I0H58X) mannitol (UNII: 3OWL53L36A) glyceryl monostearate (UNII: 230OU9XXE4) potassium cetyl phosphate (UNII: 03KCY6P7UT) trolamine (UNII: 9O3K93S3TK) polyoxyl 20 cetostearyl ether (UNII: YRC528SWUY) propylparaben (UNII: Z8IX2SC1OH) panthenol (UNII: WV9CM0O67Z) sodium pyrrolidone carboxylate (UNII: 469OTG57A2) steareth-20 (UNII: L0Q8IK9E08) ceteth-20 (UNII: I835H2IHHX) chlorphenesin (UNII: I670DAL4SZ) titanium dioxide (UNII: 15FIX9V2JP) teprenone (UNII: S8S8451A4O) polysorbate 80 (UNII: 6OZP39ZG8H) polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) edetate sodium (UNII: MP1J8420LU) butylene glycol (UNII: 3XUS85K0RA) kalmia latifolia leaf (UNII: 79N6542N18) yeast (UNII: 3NY3SM6B8U) vitamin a palmitate (UNII: 1D1K0N0VVC) ascorbyl palmitate (UNII: QN83US2B0N) serine (UNII: 452VLY9402) urea (UNII: 8W8T17847W) acacia (UNII: 5C5403N26O) allantoin (UNII: 344S277G0Z) sodium succinate anhydrous (UNII: V8ZGC8ISR3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-038-61 1 in 1 BOX 1 NDC:14783-038-62 50 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/15/2010 LBEL PARIS RENOVANCE JOUR
octinoxate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.375 g in 5 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.25 g in 5 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.15 g in 5 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) cyclomethicone 6 (UNII: XHK3U310BA) glycerin (UNII: PDC6A3C0OX) snow pea (UNII: 84SKC33B1I) c12-15 alkyl benzoate (UNII: A9EJ3J61HQ) medium-chain triglycerides (UNII: C9H2L21V7U) cyclomethicone 5 (UNII: 0THT5PCI0R) cetyl alcohol (UNII: 936JST6JCN) cetostearyl alcohol (UNII: 2DMT128M1S) isopropyl myristate (UNII: 0RE8K4LNJS) isononyl isononanoate (UNII: S4V5BS6GCX) sorbitan monostearate (UNII: NVZ4I0H58X) mannitol (UNII: 3OWL53L36A) glyceryl monostearate (UNII: 230OU9XXE4) potassium cetyl phosphate (UNII: 03KCY6P7UT) trolamine (UNII: 9O3K93S3TK) polyoxyl 20 cetostearyl ether (UNII: YRC528SWUY) propylparaben (UNII: Z8IX2SC1OH) panthenol (UNII: WV9CM0O67Z) sodium pyrrolidone carboxylate (UNII: 469OTG57A2) steareth-20 (UNII: L0Q8IK9E08) ceteth-20 (UNII: I835H2IHHX) chlorphenesin (UNII: I670DAL4SZ) titanium dioxide (UNII: 15FIX9V2JP) teprenone (UNII: S8S8451A4O) polysorbate 80 (UNII: 6OZP39ZG8H) polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) edetate sodium (UNII: MP1J8420LU) butylene glycol (UNII: 3XUS85K0RA) kalmia latifolia leaf (UNII: 79N6542N18) yeast (UNII: 3NY3SM6B8U) vitamin a palmitate (UNII: 1D1K0N0VVC) ascorbyl palmitate (UNII: QN83US2B0N) serine (UNII: 452VLY9402) urea (UNII: 8W8T17847W) acacia (UNII: 5C5403N26O) allantoin (UNII: 344S277G0Z) sodium succinate anhydrous (UNII: V8ZGC8ISR3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-028-51 1 in 1 BOX 1 NDC:14783-028-52 5 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/15/2010 LBEL PARIS RENOVANCE JOUR
octinoxate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.05 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.03 g in 1 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) cyclomethicone 6 (UNII: XHK3U310BA) glycerin (UNII: PDC6A3C0OX) snow pea (UNII: 84SKC33B1I) c12-15 alkyl benzoate (UNII: A9EJ3J61HQ) medium-chain triglycerides (UNII: C9H2L21V7U) cyclomethicone 5 (UNII: 0THT5PCI0R) cetyl alcohol (UNII: 936JST6JCN) cetostearyl alcohol (UNII: 2DMT128M1S) isopropyl myristate (UNII: 0RE8K4LNJS) isononyl isononanoate (UNII: S4V5BS6GCX) sorbitan monostearate (UNII: NVZ4I0H58X) mannitol (UNII: 3OWL53L36A) glyceryl monostearate (UNII: 230OU9XXE4) potassium cetyl phosphate (UNII: 03KCY6P7UT) trolamine (UNII: 9O3K93S3TK) polyoxyl 20 cetostearyl ether (UNII: YRC528SWUY) propylparaben (UNII: Z8IX2SC1OH) panthenol (UNII: WV9CM0O67Z) sodium pyrrolidone carboxylate (UNII: 469OTG57A2) steareth-20 (UNII: L0Q8IK9E08) ceteth-20 (UNII: I835H2IHHX) chlorphenesin (UNII: I670DAL4SZ) titanium dioxide (UNII: 15FIX9V2JP) teprenone (UNII: S8S8451A4O) polysorbate 80 (UNII: 6OZP39ZG8H) polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) edetate sodium (UNII: MP1J8420LU) butylene glycol (UNII: 3XUS85K0RA) kalmia latifolia leaf (UNII: 79N6542N18) yeast (UNII: 3NY3SM6B8U) vitamin a palmitate (UNII: 1D1K0N0VVC) ascorbyl palmitate (UNII: QN83US2B0N) serine (UNII: 452VLY9402) urea (UNII: 8W8T17847W) acacia (UNII: 5C5403N26O) allantoin (UNII: 344S277G0Z) sodium succinate anhydrous (UNII: V8ZGC8ISR3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-018-41 1 in 1 BOX 1 NDC:14783-018-42 1 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/15/2010 Labeler - Ventura International LTD (603192787)