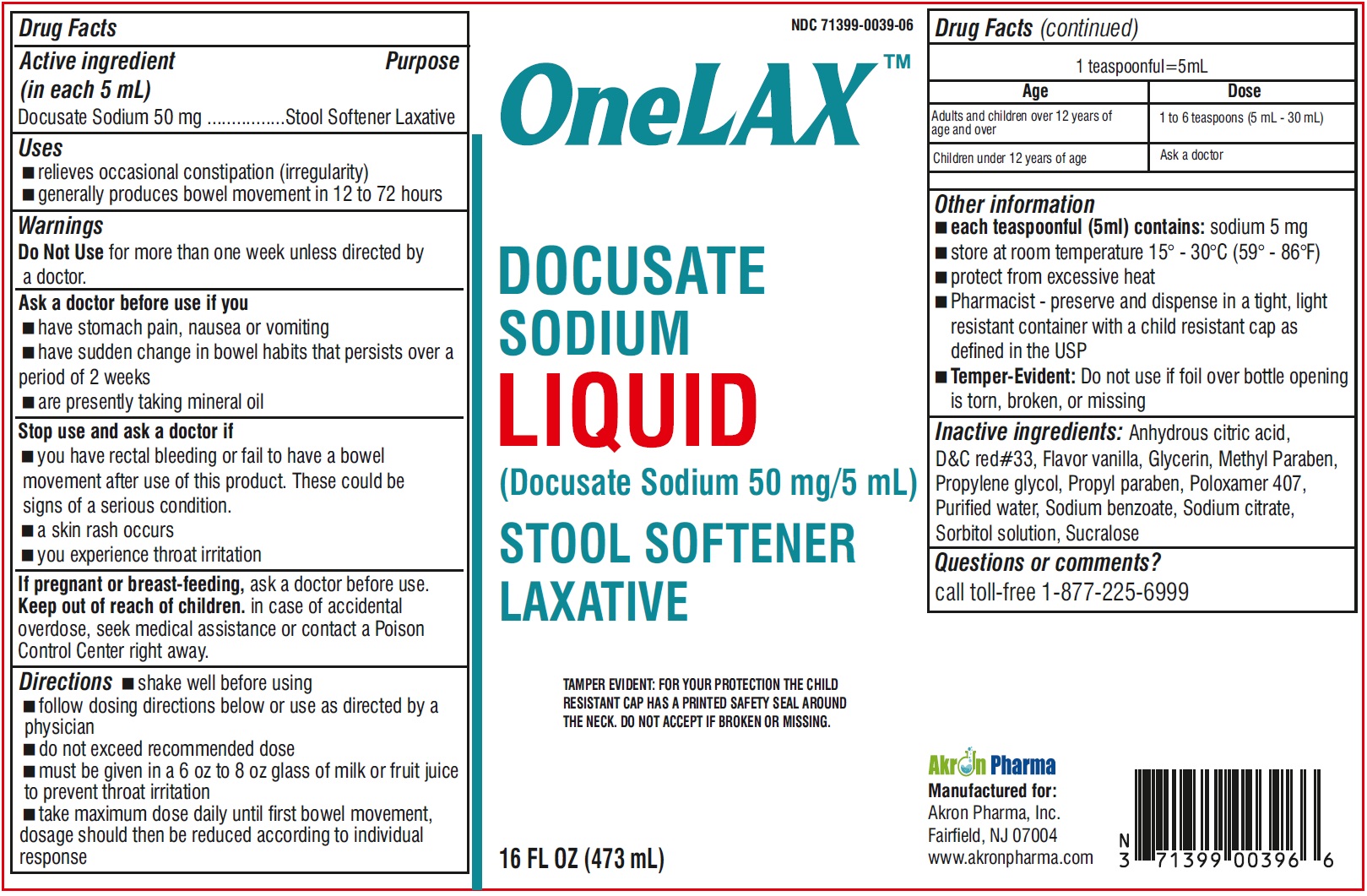
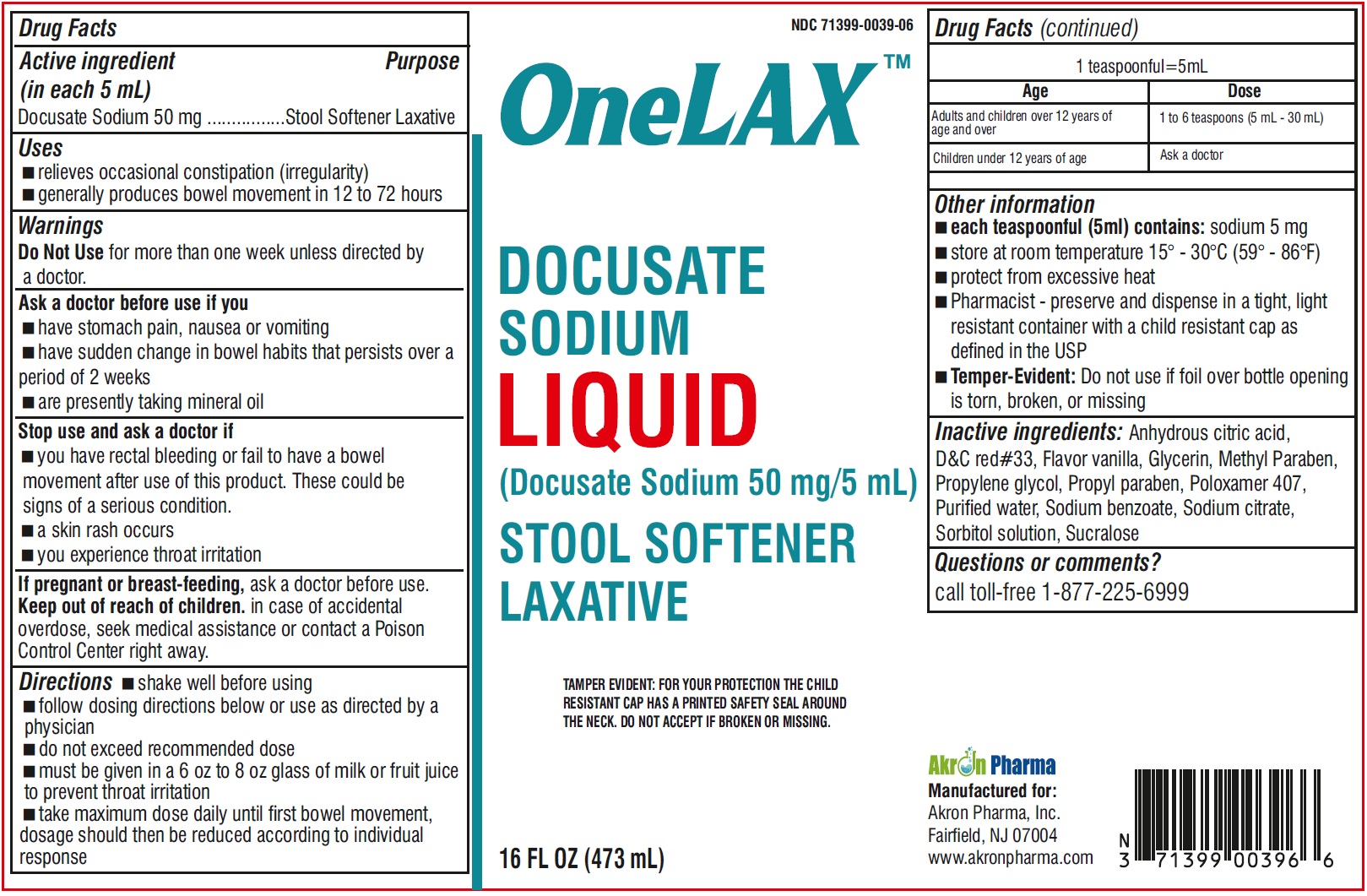
Label: ONELAX- docusate sodium liquid
- NDC Code(s): 71399-0039-6
- Packager: Akron Pharma
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active ingredient (in each 5 mL)
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you
- Stop use and ask a doctor if
- if pregnant or breast-feeding,
- Keep out of reach of children
-
Directions
- shake well before using
- follow dosing directions below or use as directed by a physician
- do not exceed recommended dose
- must be given in a 6 oz to 8 oz glass of milk or fruiit juice to prevent throat irritation
- take maximum dose daily until first bowel movement, dosage should then be reduced according to indivisual response
- DOSAGE & ADMINISTRATION
-
Other information
- wach teaspoonful (5 ml) contains: sodium 5 mg
- store at room temperature 15o - 30oC (59o - 86oF)
- protect from excessive heat
- Pharmacist-preserve and dispense in a tight, light resistant container with a child resistant cap as defined in the USP
- Temper -Evident: Do not use if foil over bottle opening is torn, broken, or missing
- Inactive ingredients:
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONELAX
docusate sodium liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71399-0039 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLOXAMER 407 (UNII: TUF2IVW3M2) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL SOLUTION (UNII: 8KW3E207O2) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71399-0039-6 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/04/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/04/2023 Labeler - Akron Pharma (067878881)