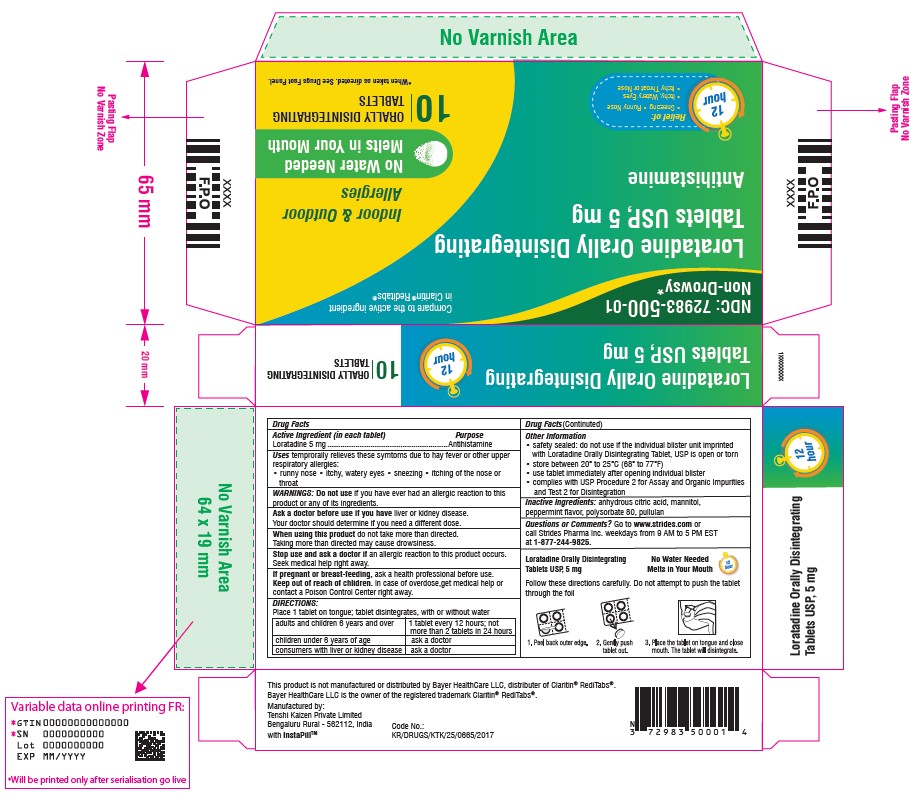
Label: LORATADINE tablet
- NDC Code(s): 72983-500-01
- Packager: Tenshi Kaizen Pvt Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
-
Other information
- safety sealed: do not use if the individual blister unit imprinted with Loratadine Orally disintegrating Tablet, USP is open or torn
- store between 20° to 25°C (68° to 77°F)
- use tablet immediately after opening individual blister
- complies with USP Procedure 2 for Assay and Organic Impurities and Test 2 for Disintegration
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72983-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PULLULAN (UNII: 8ZQ0AYU1TT) POLYSORBATE 80 (UNII: 6OZP39ZG8H) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor PEPPERMINT Imprint Code T5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72983-500-01 1 in 1 CARTON 05/19/2021 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212795 05/19/2021 Labeler - Tenshi Kaizen Pvt Ltd (675478488) Establishment Name Address ID/FEI Business Operations Tenshi Kaizen Pvt Ltd 675478488 analysis(72983-500) , manufacture(72983-500) , pack(72983-500)