Label: ATROPINE SULFATE ointment
- NDC Code(s): 24208-825-55
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 2, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
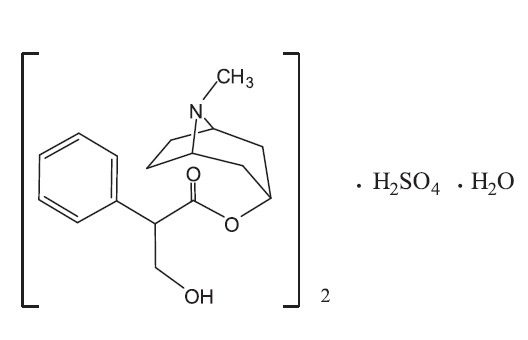
Atropine Sulfate Ophthalmic Ointment, 1% is a sterile topical anticholinergic for ophthalmic use. The active ingredient is represented by the chemical structural formula:
(C17H23NO3)2•H2SO4•H2O
Mol. Wt. 694.83Chemical Name:
Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo-[3.2.1]oct-3-yl ester, endo-(±)-, sulfate (2:1) (salt), monohydrate.
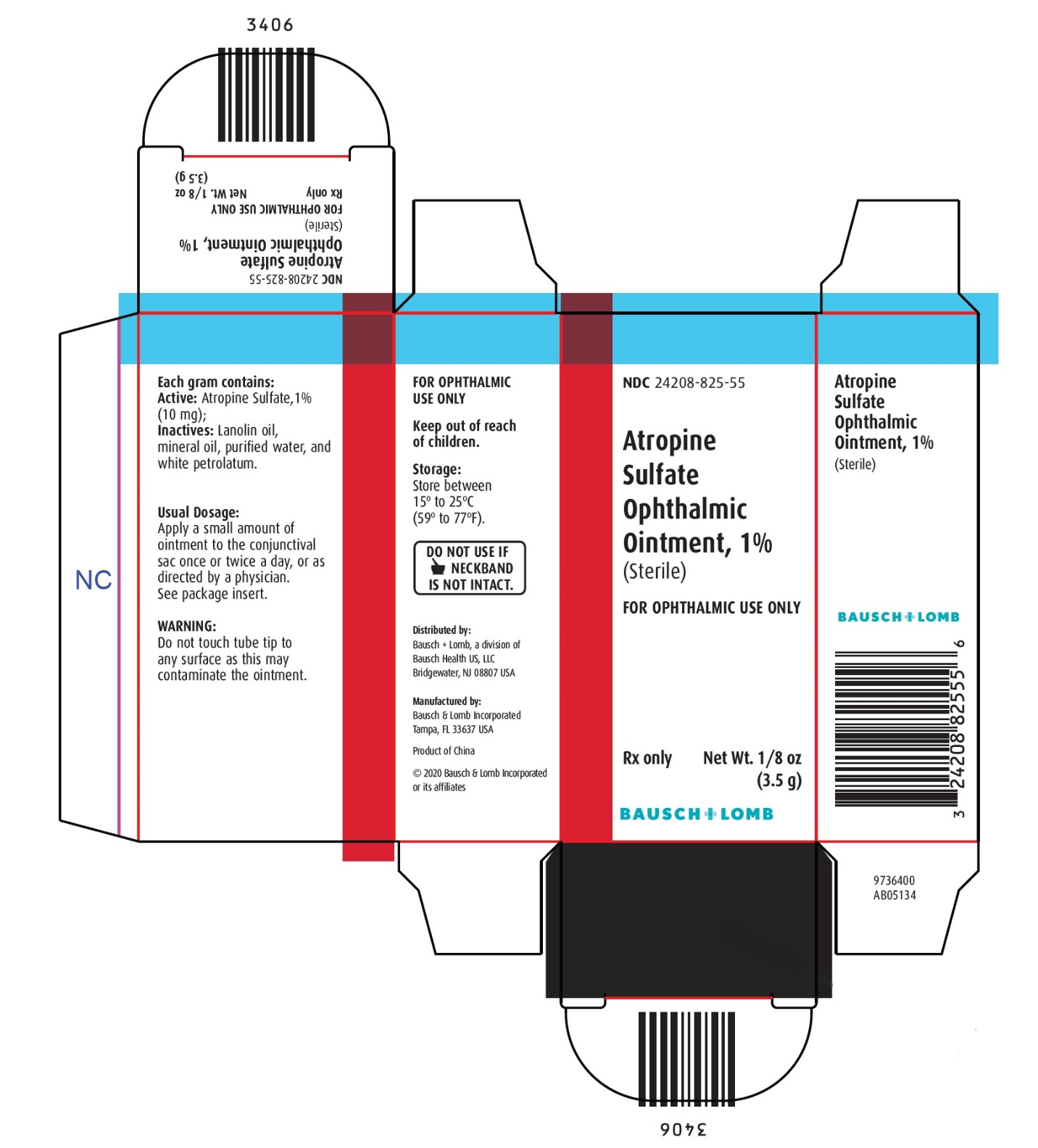
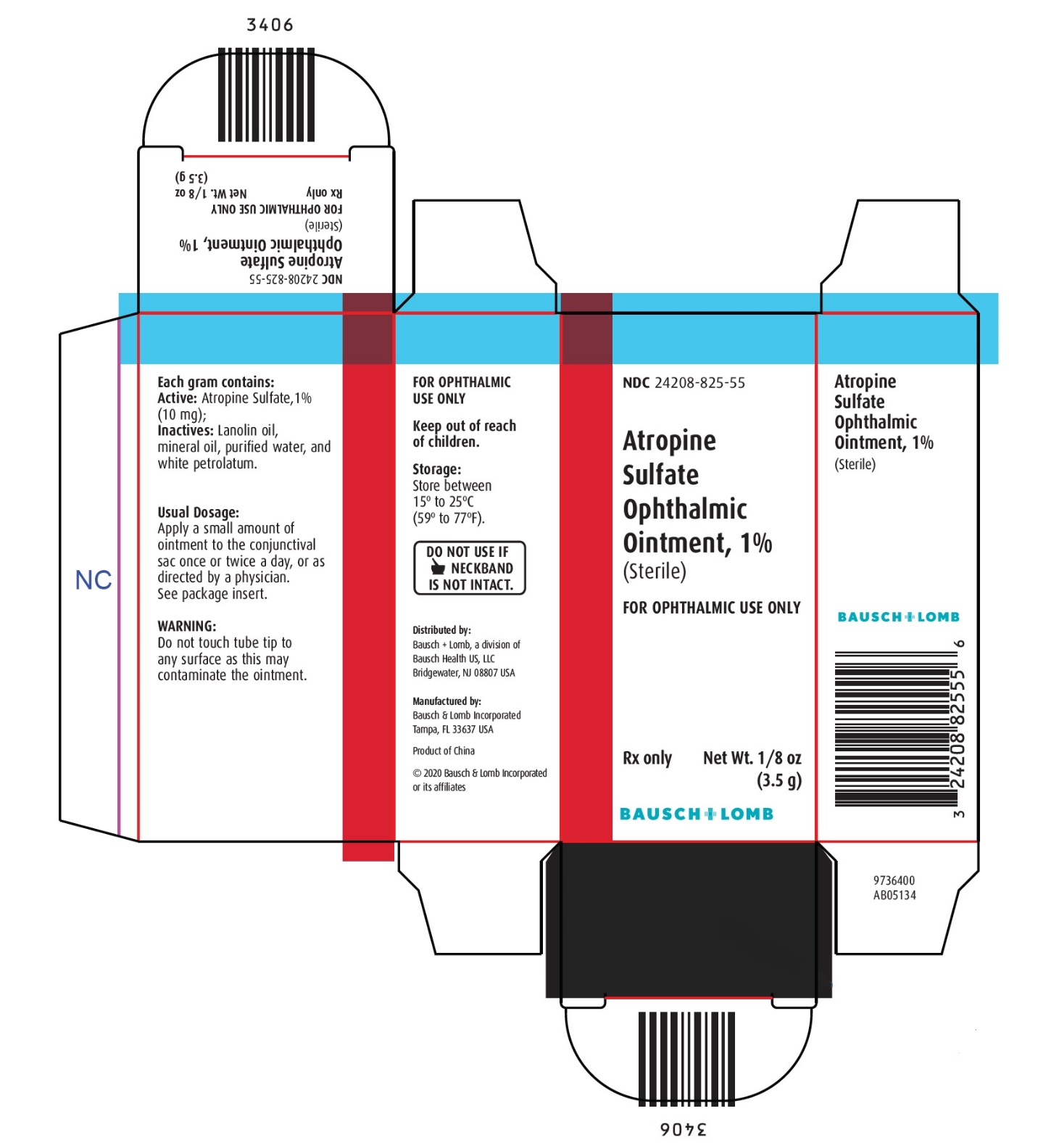
Each gram contains:
ACTIVE: Atropine Sulfate, 1% (10 mg); INACTIVES: Lanolin oil, mineral oil, purified water, and white petrolatum.
- CHEMICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
This product should not be used in patients with primary glaucoma or a predisposition to narrow anterior chamber angle glaucoma.
This product should not be used in pediatric patients who have previously had a severe systemic reaction to atropine. This product should not be used in those persons showing hypersensitivity to any component of this preparation.
-
WARNINGS
In pediatric patients, use with extreme caution. Excessive use in pediatric patients or in certain individuals with a previous history of susceptibility to belladonna alkaloids may produce systemic symptoms of atropine poisoning. If this occurs, discontinue medication and use appropriate therapy as outlined in OVERDOSAGE.
-
PRECAUTIONS
To avoid excessive systemic absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made. Administration of atropine in infants requires great caution.
Patient Warning:
Patients should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patients may experience sensitivity to light and should protect eyes in bright illumination during dilation.
Parents should be warned not to get this preparation in their children’s mouth and to wash their own hands and the child’s hands following administration.Carcinogenesis, Mutagenesis, Impairment of Fertility:
No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Animal reproduction studies have not been performed with atropine. It is also not known whether atropine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Atropine should be given to pregnant women only if clearly needed.
-
ADVERSE REACTIONS
Prolonged use may produce local irritation characterized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Severe reactions are manifested by hypotension with progressive respiratory depression. Coma and death have been reported in the very young.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Systemic atropine toxicity is manifested by flushing and dryness of the skin (a rash may be present in pediatric patients), blurred vision, a rapid and irregular pulse, fever, abdominal distension in infants, mental aberration (hallucinosis) and loss of neuromuscular coordination.
Atropine poisoning, although distressing, is rarely fatal, even with large doses of atropine, and is self-limited if the cause is recognized and the atropine medication is discontinued. In severe intoxication, physostigmine salicylate may be administered parenterally to provide more prompt relief of the intoxication. Give physostigmine salicylate as 1-5 mL IV of dilution containing 1 mg in 5 mL of saline. The smaller dose is for pediatric patients, and injection should take not less than 2 minutes. EKG control is advisable. Dosage can be repeated every 5 minutes up to a total dose of 2 mg in pediatric patients and 6 mg in adults every 30 minutes. Physostigmine is contraindicated in hypotensive reactions. Atropine (1 mg) should be available for immediate injection if physostigmine causes bradycardia, convulsions or bronchoconstriction. In pediatric patients, the body surface must be kept moist.
Use extreme caution when employing short-acting barbiturates to control excitement. - DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
-
STORAGE:
Store between 15° to 25°C (59° to 77°F).
Keep out of reach of children.
Distributed by:
Bausch + Lomb, a division of Bausch Health US, LLC
Bridgewater, NJ 08807 USAManufactured by:
Bausch & Lomb Incorporated, Tampa, FL 33637 USA
© 2020 Bausch & Lomb Incorporated or its affiliates
Rev. 04/2020
9115203 (Folded)
9115303 (Flat) - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ATROPINE SULFATE
atropine sulfate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24208-825 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN OIL (UNII: OVV5IIJ58F) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24208-825-55 1 in 1 CARTON 09/30/1990 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 09/30/1990 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(24208-825) , LABEL(24208-825) , PACK(24208-825)